• How key toxicity-associated nanomaterial properties impact nano-bio interactions is discussed.
• Analytical methods for studying nano-bio interactions are presented.
• Current regulatory and legislative frameworks regulating nanomaterials are introduced.
• Challenges facing nanomaterials’ safety evaluation and possible solutions are given.
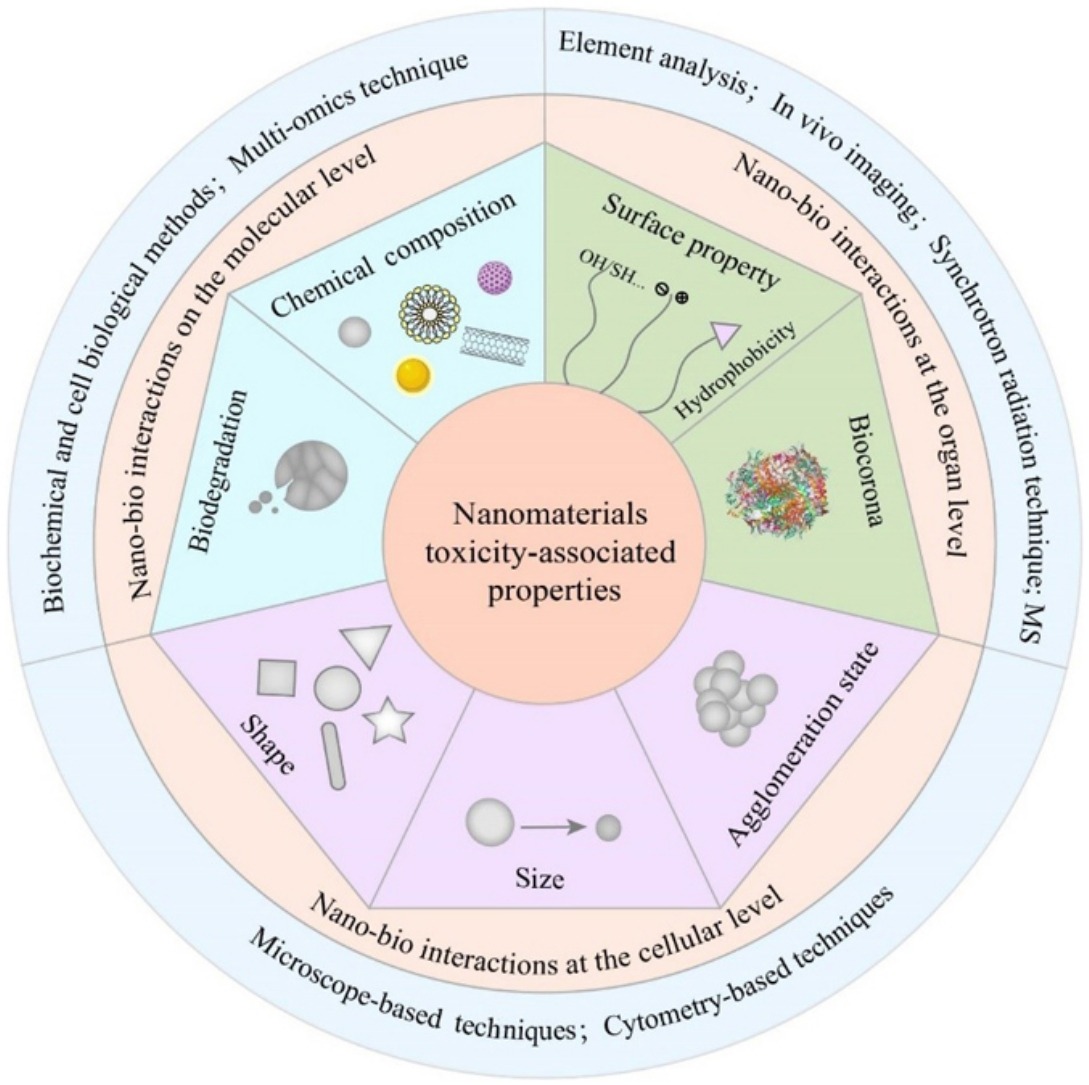
Manufactured nanomaterials with unique properties have been extensively applied in various industrial, agricultural or medical fields. However, some of the properties have been identified to be closely related to nanomaterial toxicity. The “nano-paradox” has aroused concerns over the use and development of nanotechnology, which makes it difficult for regulatory agencies to regulate nanomaterials. The key to fulfilling proper nanomaterial regulation lies in the adequate understanding of the impact of nanomaterial properties on nano-bio interactions. To this end, we start the present work with a brief introduction to nano-bio interactions at different levels. Based on that, how key toxicity-associated properties of manufactured nanomaterials (i.e., size, shape, chemical composition, surface properties, biocorona formation, agglomeration and/or aggregation state, and biodegradability) impact their toxicokinetics, cellular uptake, trafficking and responses, and toxicity mechanisms is deeply explored. Moreover, advanced analytical methods for studying nano-bio interactions are introduced. Furthermore, the current regulatory and legislative frameworks for nanomaterial-containing products in different regions and/or countries are presented. Finally, we propose several challenges facing the nanotoxicology field and their possible solutions to shed light on the safety evaluation of nanomaterials.