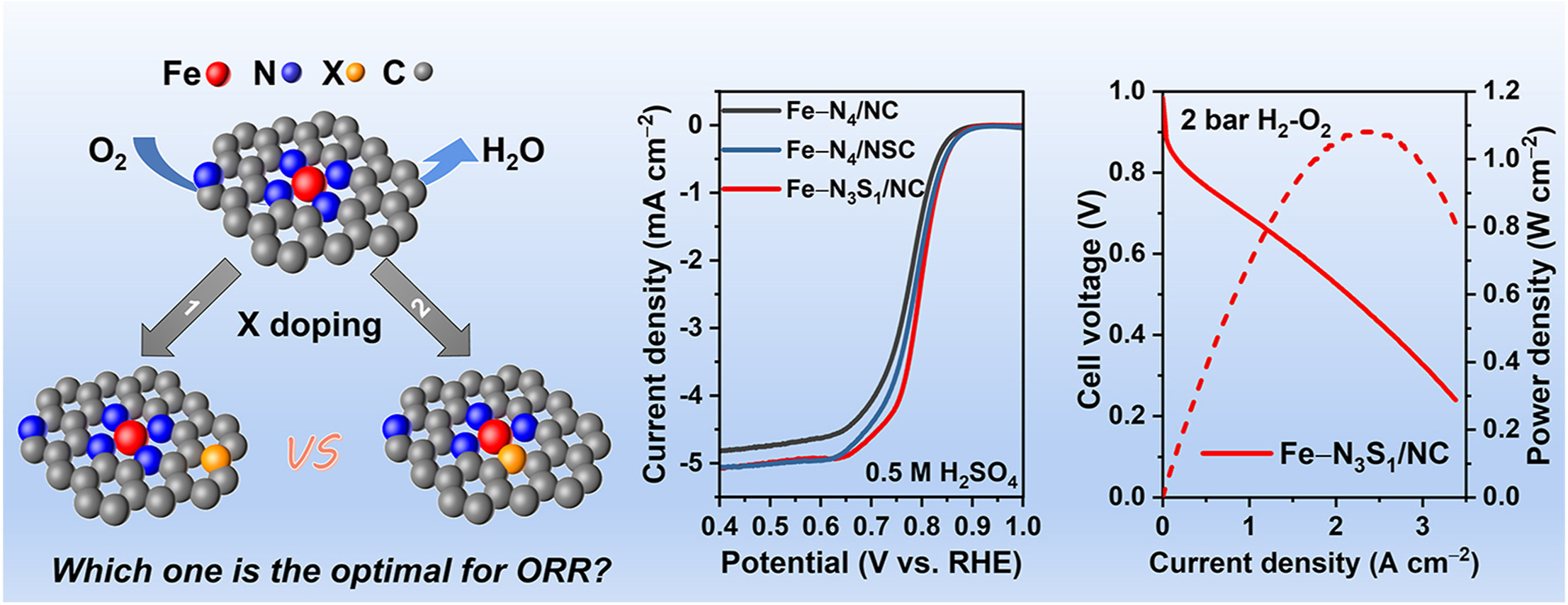
• Chalcogen atom doping improves the oxygen reduction reaction activity and stability of Fe–N–C catalysts.
• The doping positions are successfully engineered via different doping sequences.
• The first coordination shell of the center iron site is identified as the optimal doping position.
• The optimized Fe–N3S1/NC catalyst exhibits an outstanding peak power density of 1.08 W cm−2 in real fuel cells.
The excellent oxygen reduction reaction (ORR) activity of Fe–N–C catalysts in acidic media makes them potential for low-cost proton exchange membrane fuel cells. In recent years, it has been shown that heteroatoms (B, O, S, P, Cl, F, etc.) can be used as electron-withdrawing groups to modulate the planar structure and electron distribution of the Fe–Nx active sites to achieve simultaneous improvement of catalytic activity and stability. However, the optimal location of the heteroatoms remains unclear. Here, taking chalcogen heteroatoms (S and Se) as an example, we control the doping positions and investigate their effect on the ORR performance of the Fe–N–C catalysts. The first coordination shell of the iron single atom is identified as the optimal doping position. The optimized catalysts Fe–N3S1/NC and Fe–N3Se1/NC demonstrate improved activity and stability in both half cells and fuel cells. This work provides insights into the enhancement mechanism of heteroatom doping in single-atom catalysts.