-
Volumes 84-95 (2024)
-
Volume 92
Pages 1-316 (September 2024)
-
Volume 91
Pages 1-378 (August 2024)
-
Volume 90
Pages 1-580 (July 2024)
-
Volume 89
Pages 1-278 (June 2024)
-
Volume 88
Pages 1-350 (May 2024)
-
Volume 87
Pages 1-338 (April 2024)
-
Volume 86
Pages 1-312 (March 2024)
-
Volume 85
Pages 1-334 (February 2024)
-
Volume 84
Pages 1-308 (January 2024)
-
Volume 92
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
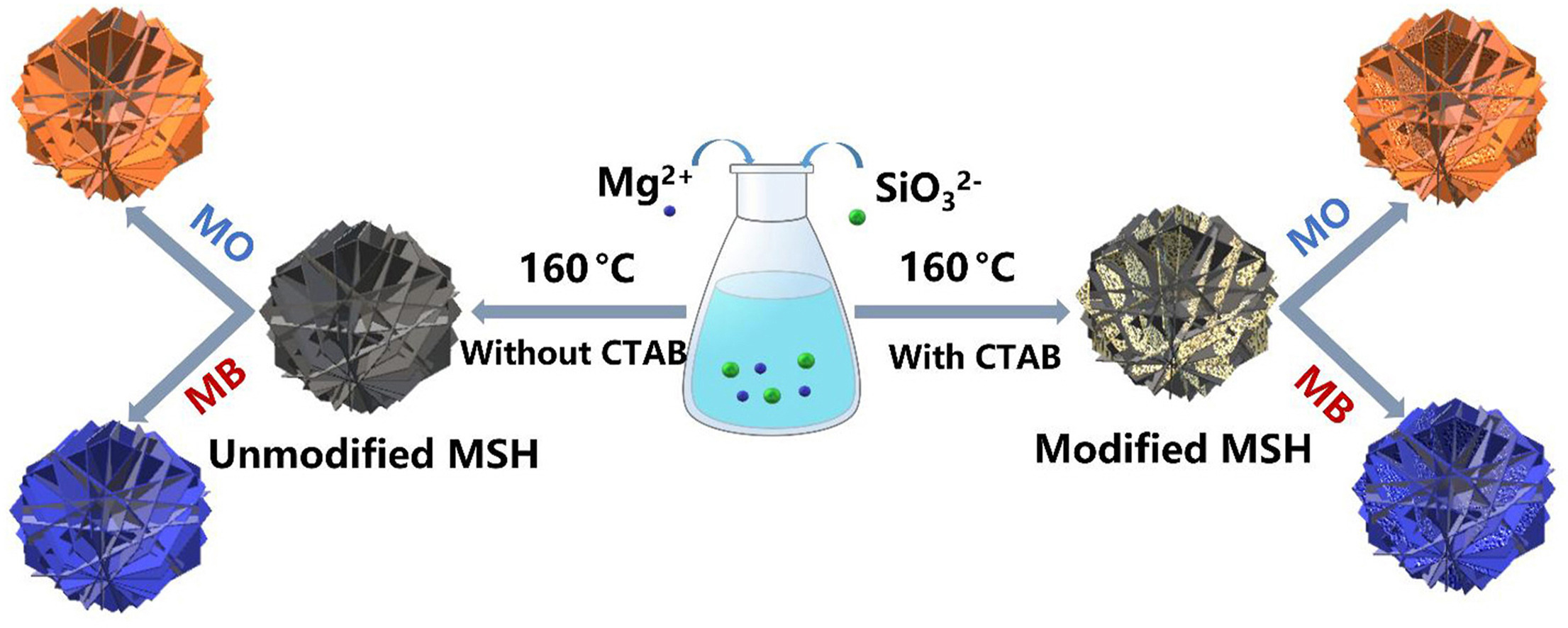
• Hierarchical flower-like magnesium silicate hydrate microspheres were fabricated.
• Formation mechanism and adsorption behavior of microspheres has been investigated.
• Methyl orange removal follows CTAB, and methylene blue adsorption is related to Si.
Hierarchical porous magnesium silicate hydrate (MSH) microspheres composed of sheets are successfully developed under facile conditions using a hard template. The role of hexadecyltrimethylammonium bromide (CTAB) on the formation and adsorption behavior was also observed for the methyl orange and methylene blue. The formed MSH possesses a surface area of 453.24 m2/g, an average pore size of 6.38 nm, and a pore volume of 0.75 cm3/g without CTAB. Based on the role of CTAB and the change in the ratio of Mg/Si, the MSH retained its sphere-like structure with a variation in pore parameters. The formed MSH was used as an adsorbent to remove methylene blue and methyl orange. The pseudo-second-order kinetic and Langmuir Isotherm models are well-fitted, with a 256.4 mg/g removal capacity and 84.2 mg/g for methylene blue and methyl orange, respectively. The modified MSH with CTAB played a positive role for the methyl orange and a negative role for the methylene blue regarding removal performance.