- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
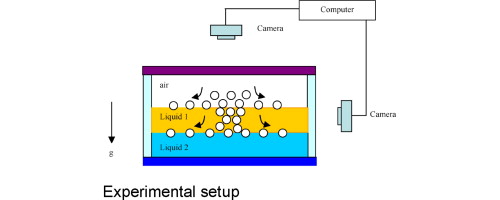
This paper is concerned with the dispersion of particles on the fluid–liquid interface. In a previous study we have shown that when small particles, e.g., flour, pollen, glass beads, etc., contact an air–liquid interface, they disperse rapidly as if they were in an explosion. The rapid dispersion is due to the fact that the capillary force pulls particles into the interface causing them to accelerate to a large velocity. In this paper we show that motion of particles normal to the interface is inertia dominated; they oscillate vertically about their equilibrium position before coming to rest under viscous drag. This vertical motion of a particle causes a radially-outward lateral (secondary) flow on the interface that causes nearby particles to move away. The dispersion on a liquid–liquid interface, which is the primary focus of this study, was relatively weaker than on an air–liquid interface, and occurred over a longer period of time. When falling through an upper liquid the particles have a slower velocity than when falling through air because the liquid has a greater viscosity. Another difference for the liquid–liquid interface is that the separation of particles begins in the upper liquid before the particles reach the interface. The rate of dispersion depended on the size of the particles, the densities of the particle and liquids, the viscosities of the liquids involved, and the contact angle. For small particles, partial pinning and hysteresis of the three-phase contact line on the surface of the particle during adsorption on liquid–liquid interfaces was also important. The frequency of oscillation of particles about their floating equilibrium increased with decreasing particle size on both air–water and liquid–liquid interfaces, and the time to reach equilibrium decreased with decreasing particle size. These results are in agreement with our analysis.