- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
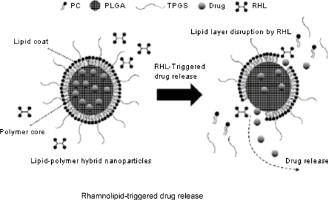
► Poly(lactic-co-glycolic acid) and phosphatidylcholine are used as the polymer nanoparticle core and lipid coat for the hybrid nanoparticles.
► The rhamnolipid-triggered release capability of the hybrid nanoparticles enables the targeted release of the encapsulated BCS Class III drug in the vicinity of biofilm colonies.
► The rate of the triggered release can be controlled by incorporating an additional lipid layer on the hybrid nanoparticles.
In lung biofilm infection therapies, the use of lipid-polymer hybrid nanoparticles to encapsulate drugs has emerged as a promising alternative to using liposomes because they have superior physicochemical stability and still possess the biofilm affinity and sputum-penetrating ability of liposomes. To be deemed equally efficacious as liposomes against bacterial biofilms, however, the capability of hybrid nanoparticles to target-release encapsulated drugs at biofilm colonies must be demonstrated. This communication details our investigations into the trigger-release characteristics of hybrid nanoparticles in response to encountering rhamnolipids, which are ubiquitously present in biofilm colonies of Pseudomonas aeruginosa, a major respiratory pathogen. Poly(lactic-co-glycolic acid) and phosphatidylcholine were used as the polymer nanoparticle core and lipid coat, respectively. These investigations were performed using compounds from various biopharmaceutical classification systems (BCS) that differ in their lipid-membrane permeabilities. The release of BCS Class III compounds, which have poor lipid-membrane permeabilities, was successfully triggered by rhamnolipids at a concentration approximately equal to their clinically observed value, and this release was attributed to the disruption of lipid coats by rhamnolipid micelles. Not unexpectedly, BCS Class I compounds, which have high lipid-membrane permeabilities, were released freely whether or not rhamnolipids were present. The rate of the triggered release can be controlled by incorporating an additional lipid layer on the hybrid nanoparticles via the electrostatically driven adsorption of lipid vesicles.