- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
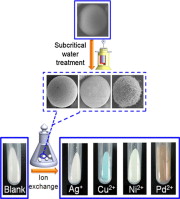
► Porous glass beads with an egg-shell structure were prepared in a batch reactor.
► The shell morphology can be easily tailored to be pores, flakes or fibers by changing the treating temperature and time.
► The prepared porous glass beads demonstrated advantages in ion sorption properties.
A subcritical water treatment method was developed for preparing porous-surfaced glass beads with an egg-shell structure in a batch reactor. Based on the “corrosion-ion-migration-recondensation” strategy, ordinary soda-lime glass beads with a diameter of about 100 μm were made first to react with subcritical water to effect controlled quantity of silicate dissolution of glass by adjusting treatment time and temperature. The dissolved silicate was then made to recondense on the glass core to form different porous shell morphologies: pores, flakes and fibers. Among these, glass beads coated with fibers with surface area of 154.5 m2/g, pore volume of 0.27 cm3/g and pore size of 7.1 nm were obtained at 573 K after 2 h of treatment. The prepared porous-surfaced glass beads were then used as adsorbent for heavy metal ions, showing various ion exchange properties. Glass beads covered with fibers displayed fast kinetics and high sorption capacity because of their egg-shell structure and high surface area. More than 90% of copper ions were adsorbed within 100 min from a solution with an initial concentration of 110 mg/L at 313 K. Ion sorption capacities were 149.33, 81.33 and 42.96 mg/g respectively for Ag+, Cu2+ and Ni2+ at 313 K. A green and low-cost method was thus developed to produce egg-shell-structured porous glass with high sorption capacity.