- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
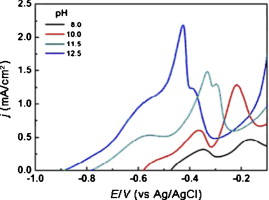
► The high pH values (10–12.5) promote the oxidation of DMAB, and suppress the reduction of the copper ion.
► Results for a dual-chelating agent system indicate that 1,5,8,12-tetraazadodecane plays an important role in chelation.
► The main effect of TEA is adsorption on copper surfaces to inhibit DMAB oxidation and to promote deposition.
Electroless copper plating was studied using dimethylamine borane (DMAB) as reductant and 1,5,8,12-tetraazadodecane as additive and triethanolamine (TEA) as buffer. The effects of pH, temperature and concentrations of reactants and additives on the anodic oxidation of DMAB and the cathodic reduction of copper ion were investigated. Experimental results indicate that high pH values (10–12.5) promote the oxidation of DMAB, and suppress the reduction of the copper ion, while high bath temperatures (55–70 °C) accelerate both anodic oxidation and cathodic reduction. Increase of the Cu2+ and DMAB concentrations can improve the deposition rate of copper plating. Results for a dual-chelating-agent system indicate that 1,5,8,12-tetraazadodecane plays an important role in chelation, while the main effect of TEA is adsorption on copper surfaces to inhibit DMAB oxidation and to promote deposition.