- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
► Potassium sodium niobate powders were synthesized by a modified sol–gel method.
► The grains become homogeneous when the K/Na molar ratio is 1.2.
► The optimum temperature to get better crystallization is 850 °C.
► The particle size of the calcined KNN powder is around 200–300 nm.
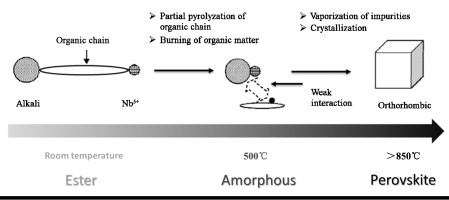
Potassium sodium niobate (KNN) powders were synthesized by a modified sol–gel method, using as starting chemicals potassium carbonate, sodium carbonate, and niobium hydroxide, and, as esterification and chelating agents, respectively, ethylene glycol (EG) and ethylene diamine tetraacetic acid (EDTA)/citrate. The effects of citric acid (CA), EG, and EDTA on the stability of the precursor sol were systemically investigated. The powders and gels were characterized by X-ray diffraction, scanning electron microscopy, Fourier transform infrared spectroscopy, and thermogravimetric analysis-differential scanning calorimetry (TGA-DSC). The results indicated that a stable precursor sol was formed when n(CA):n(Mn+) = 3:1, n(EDTA):n(NH4OH) = 1:3.5, and n(CA):n(EG) = 1:2. The xerogel was calcined at 500–950 °C to prepare the KNN powder. Pure KNN perovskite phase with a cube-like structure was synthesized at 850 °C from the precursor sol for a K/Na molar ratio of 1.2. The formation mechanism of the KNN perovskite phase was also discussed.