- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
► LiNi0.5Mn1.5O4 with cubic spinel structure was synthesized by urea combustion synthesis.
► The structure and particle-size of LiNi0.5Mn1.5O4 can be adjusted by the amount of urea and heat-treatment temperature.
► LiNi0.5Mn1.5O4 shows the largest discharge capacity of 133.6 mAh/g and capacity retention rate of 99.6% after 20 cycles.
LiNi0.5Mn1.5O4 was synthesized by combustion synthesis (UCS) using urea as fuel. X-ray diffraction and scanning electron microscope measurements showed that the spinel structure LiNi0.5Mn1.5O4 with the space group 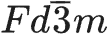 was formed during urea combustion. Both structure and particle size could be adjusted by the amount of urea and the heat treatment temperature used in the UCS. For the LiNi0.5Mn1.5O4 sample prepared with a urea/Li molar ratio of 0.57 and a heat treatment temperature of 900 °C, the particle-size distribution fell in a narrow range of 1–2 μm. Electrochemical tests indicated that this LiNi0.5Mn1.5O4 sample delivered a discharge capacity of 133.6 mAh/g with a capacity retention rate of 99.6% after 20 cycles at 0.5 C.
was formed during urea combustion. Both structure and particle size could be adjusted by the amount of urea and the heat treatment temperature used in the UCS. For the LiNi0.5Mn1.5O4 sample prepared with a urea/Li molar ratio of 0.57 and a heat treatment temperature of 900 °C, the particle-size distribution fell in a narrow range of 1–2 μm. Electrochemical tests indicated that this LiNi0.5Mn1.5O4 sample delivered a discharge capacity of 133.6 mAh/g with a capacity retention rate of 99.6% after 20 cycles at 0.5 C.