- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
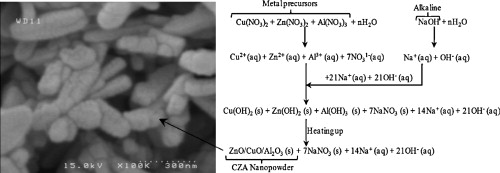
► CuO/ZnO/Al2O3 (CZA) nanopowder was synthesized hydrothermally with atomic ratio of 6:3:1.
► Relative crystallinity increased with increasing crystallization time.
► The optimal crystallization time is 6 h with crystallite size of 20 nm.
► EDX mapping indicated homogenous dispersion of elements.
A hydrothermal method was successfully used for synthesis of CuO/ZnO/Al2O3 (CZA) nanopowder with atomic ratio of 6:3:1. The effect of crystallization time (3, 6, 9, and 12 h) on physicochemical properties of nanopowder was investigated. Nanopowders were characterized using XRD, FESEM, EDX, FTIR, TG, and BET techniques. The XRD patterns confirmed metal oxides formation and their good crystallinity with average crystallite size of 20 nm as obtained by the Scherrer equation. Relative crystallinity was shown to increase with increasing crystallization time. In agreement with XRD results, FESEM images also illustrated nanosized particles. EDX mapping indicated homogenous dispersion of elements. BET specific surface area analysis showed acceptable surface area for CZA nanopowder. FTIR spectroscopy confirmed metal oxides formation during hydrothermal and calcination processing. TG results illustrated high thermal stability of the synthesized nanopowders. TG-DTG and FTIR analyses were used to propose a reaction mechanism for nanopowder formation during processing. Physicochemical characterization showed optimal crystallization time to be 6 h.