- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
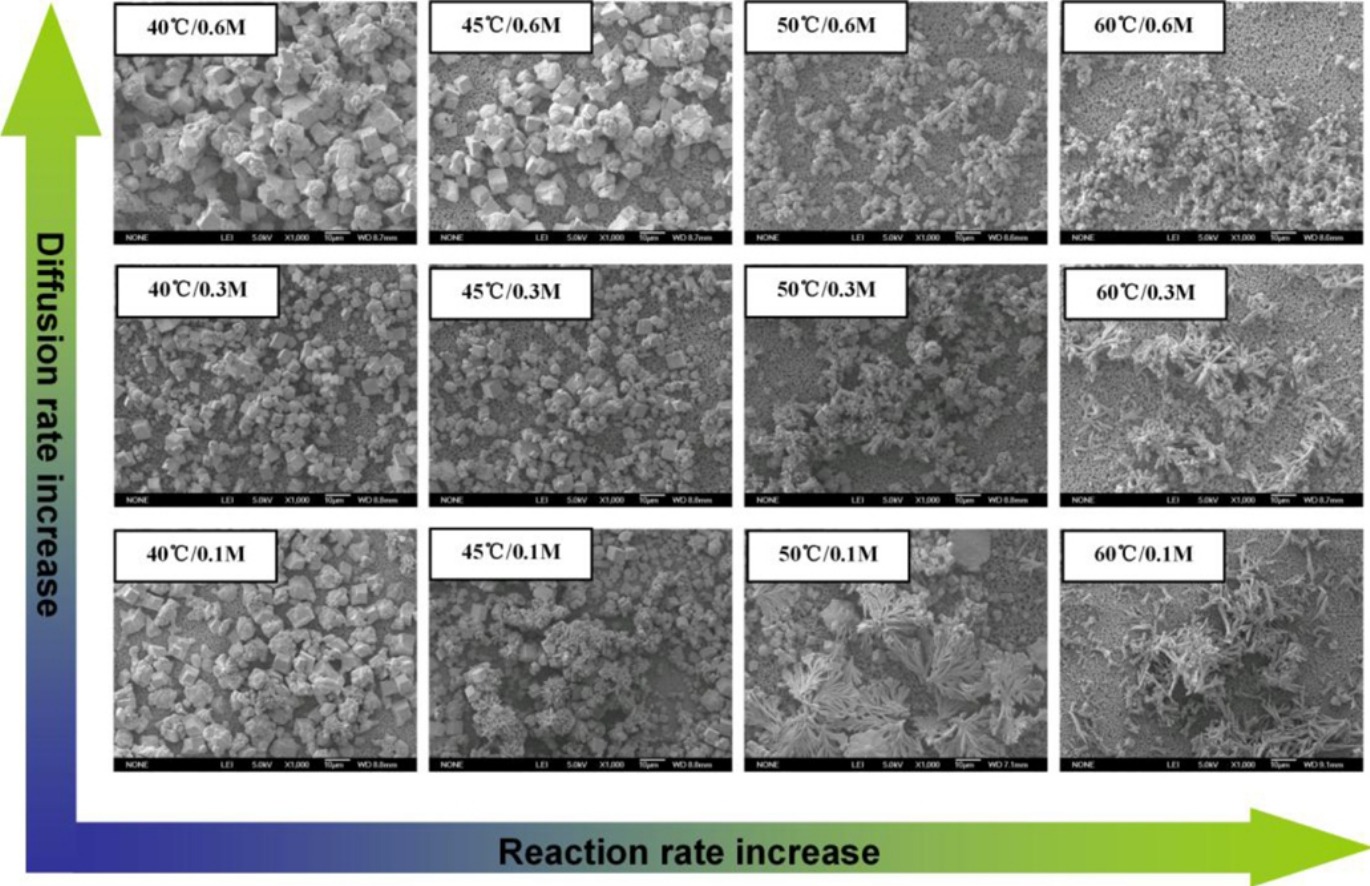
► A three-cell reactor was devised to study influence of diffusion on polymorphism of CaCO3.
► Compromise of diffusion and reaction dominated the morphology of crystallization products.
Diffusion is seldom considered by chemists and materialists in the preparation of materials while it plays an important role in the field of chemical engineering. If we look at crystallization at the atomic level, crystal growth in a solution starts from the diffusion of ions to the growing surface followed by the incorporation of ions into its lattice. Diffusion can be a rate determining step for the growth of crystals. In this paper, we take the crystallization of calcium carbonate as an example to illustrate the microscopic processes of diffusion and reaction and their compromising influence on the morphology of the crystals produced. The diffusion effect is studied in a specially designed three-cell reactor. Experiments show that a decrease of diffusion leads to retardation of supersaturation and the formation of a continuous concentration gradient in the reaction cell, thus promoting the formation of cubic calcite particles. The reaction rate is regulated by temperature. Increase of reaction rate favors the formation of needle-like aragonite particles. When diffusion and reaction play joint roles in the reaction system, their compromise dominates the formation of products, leading to a mixture of cubic and needle-like particles with a controllable ratio. Since diffusion and reaction are universal factors in the preparation of materials, the finding of this paper could be helpful in the controlled synthesis of other materials.