- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
• Ce–Mn oxides for catalytic oxidation of benzene were synthesized by flame spray pyrolysis (FSP).
• Ce–Mn oxides of size <40 nm and specific surface areas of 20–50 m2/g were formed with different Ce–Mn ratios.
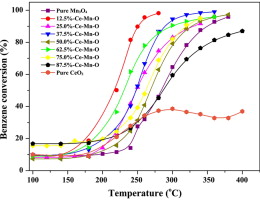
• Relative lower benzene conversion temperature (T95 ≈ 260 °C) was achieved by 12.5%-Ce–Mn oxides.
• Better activity was attracted to the synergetic effect of Ce and Mn and small particle sizes as well.
Flame spray pyrolysis (FSP) was utilized to synthesize Ce–Mn oxides in one step for catalytic oxidation of benzene. Cerium acetate and manganese acetate were used as precursors. The materials synthesized were characterized using X-ray diffraction (XRD), N2 adsorption, X-ray photoelectron spectroscopy (XPS), transmission electron microscopy (TEM), Raman spectroscopy, and H2-temperature programmed reduction (H2-TPR) and their benzene catalytic oxidation behavior was evaluated. Mn ions were evidenced in multiple chemical states. Crystalline Ce–Mn oxides consist of particles with size <40 nm and specific surface areas (SSA) of 20–50 m2/g. Raman spectrums and H2-TPR results indicated the interaction between cerium and manganese oxides. Flame-made 12.5%-Ce–Mn oxide exhibited excellent catalytic activity at relatively low temperatures (T95 about 260 °C) compared to other Ce–Mn oxides with different cerium-to-manganese ratios. Redox mechanism and strong interaction conform to structure analysis that Ce–Mn strong interaction formed during the high temperature flame process and the results were used to explain catalytic oxidation of benzene.