- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
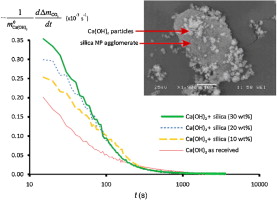
► Synthetic CO2 adsorbent is made by dry mixing of Ca(OH)2 and nanosilica powders.
► Acting as dispersant for Ca(OH)2, nanosilica enhances contact efficiency between CO2 and Ca(OH)2.
► Addition of nanosilica helps increase CO2 adsorption rate during fast carbonation stage.
Promising technologies have recently emerged to capture CO2 from postcombustion flue gas and to enhance the production of hydrogen from natural gas by steam-methane reforming, on the basis of sorption of CO2 by Ca-based powders. The rate of CO2 sorption on Ca-based powders is limited by both carbonation kinetics and transport of CO2 to unreacted sorption sites. Ca-based powders may exhibit cohesive aggregation, thus hindering gas–solids contact efficiency. In our work, we tested the sorption rate of powder samples prepared by dry mixing of a cohesive Ca(OH)2 powder with a silica nanopowder used as additive. The silica nanopowder serves to improve the dispersibility of Ca(OH)2. Consequently, when a CO2 enriched gas and the modified sorbent are brought into contact, the rate of CO2 sorption is enhanced in the initial fast phase of interest for practical applications.