- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
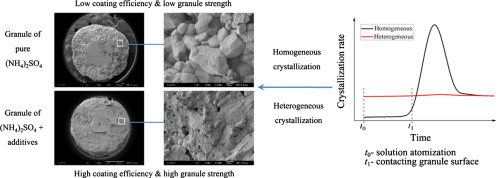
► High efficiency of (NH4)2SO4 aqueous solution coating granulation was achieved.
► Sand-like structure in the granules of pure (NH4)2SO4 was found.
► Compact structure of granules was achieved by adding CaCO3 or SiO2 additives.
► Granules with higher strength were produced from heterogeneous crystallization.
Spherical 2–4 mm granules of ammonium sulfate (NH4)2SO4 are promising fertilizer for practical use, though only much smaller grains are being produced in industry. This work used coating granulation to produce large spherical granules of (NH4)2SO4 in a fluidized bed by spraying its aqueous solution onto 0.9–1.6 mm (NH4)2SO4 core particles. However, the overall coating efficiency was only 58% due to loss as dust by attrition of (NH4)2SO4 in the vent gas. By adding CaCO3 or SiO2 particles into the feed solution, the coating efficiency was increased to over 90%. This increase in coating efficiency was due to a change in the crystallization mechanism of (NH4)2SO4. The added CaCO3 or SiO2 particles provided a heterogeneous surface that induced (NH4)2SO4 to crystallize uniformly to form a more compact structure less susceptible to attrition.