- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
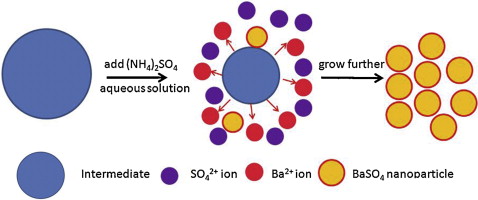
• BaSO4 nanoparticles were synthesized with simple precipitation in the presence of PAAS.
• Intermediates derived from the interaction of PAAS and Ba2+ play a role as control releasing agent.
• The method yields BaSO4 nanoparticles with narrow distribution and good dispersibility.
Well-dispersed BaSO4 nanoparticles were synthesized in the presence of sodium polyacrylate (PAAS) by a simple precipitation method, with BaCl2 and (NH4)2SO4 as reactants. The different roles performed by PAAS in the synthesis of BaSO4 nanoparticles were investigated using X-ray diffractometry, Fourier transform infrared spectroscopy, and transmission electron microscopy. The results indicate that the as-synthesized BaSO4 nanoparticles were spheres with an average diameter of 30 nm and that their surfaces were affected by the PAAS. Under a typical procedure employed, PAAS reacted with BaCl2 to yield an intermediate, serving as a control releasing agent and separating the nucleation and crystal growth processes of the BaSO4 nuclei. During formation of the BaSO4 nanospheres, the intermediate slowly dissolved and released barium and polyacrylate ions, inhibiting the growth and aggregation of newly formed BaSO4 seeds and resulting in particles of narrow diameter distribution and improved dispersibility. Moreover, these polyacrylate ions further modified the surfaces of the BaSO4 nanoparticles.