- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
► Carbon supported Pt, PtCo, PtRu catalysts are subjected to heat-treatment.
► Heating induced structural changes of the catalysts were investigated.
► Different performance of heated PtCo, PtRu catalysts is likely due to difference in atomic ratios.
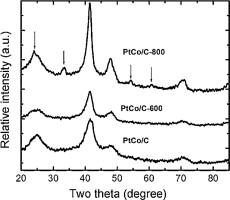
Commercially available carbon-supported Pt, PtCo and PtRu catalysts from E-TEK are heat-treated in turn at 600 °C and 800 °C each for an hour. The as-received and as-heated catalysts are used as anode catalysts for direct methanol fuel cells. Structural and surface composition changes induced by heating are analyzed by X-ray diffraction (XRD) and X-ray photoelectron spectroscopy (XPS), respectively. For the Pt catalyst, heating the catalysts caused only the mass activity decrease due to particle sintering, whereas the specific activity and CO tolerance remained unchanged. The performance of the PtCo and PtRu catalysts is affected differently by heating. Heating the PtRu catalyst adversely affects its catalytic activity and its CO tolerance due to Pt depletion at the surface. In contrast, although Pt depletion also takes place for the heated PtCo catalysts, these catalysts show an even higher specific activity and approximately the same CO tolerance. The observed difference is likely due to the optimum atomic ratio difference for Ru/Pt and Co/Pt; an increased atomic ratio on the surface for Co/Pt results in an activity enhancement, which is contrary to the effect of the increase of Ru/Pt atomic ratio.