- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
► A facile route to synthesize MnO2/MWCNTs was devised in aqueous solution under ambient conditions.
► Composite of MWCNTs loaded with 20 wt%MnO2 nanosheets has a high specific capacitance of 234 F/g.
► 80% of the initial capacitance of the MnO2/MWCNTs (20 wt%) is maintained after 1000 charge–discharge cycles.
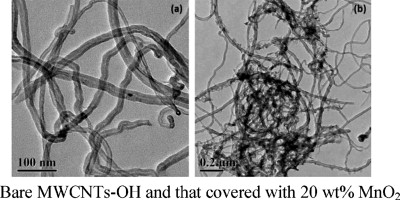
The poor electrical conductivity of MnO2 limits its use as an electrode material. To overcome this limitation, we report an easy and rapid approach to deposit nanosized MnO2 onto multi-wall carbon nanotubes functionalized with hydroxyl groups (MWCNTs-OH) by chemical reduction of KMnO4 with MnSO4 in aqueous solution under ambient conditions. Characterization with XRD and TEM reveals that the obtained MnO2/MWCNTs-OH composite is nanocrystalline and partially covered by MnO2 nanosheets with a thickness of 1–3 nm at a MnO2 loading of 20 wt%. Cyclic voltammetry (CV) and galvanostatic charge–discharge measurements reveal that the MnO2/MWCNTs-OH composite with a MnO2 loading of 20 wt% has a relatively high specific capacitance of 234 F/g at a scan rate of 2 mV/s and exhibits good cycling stability. Furthermore, the oxygen reduction reaction (ORR) shows that MnO2/MWCNTs-OH composite may have potential applications as a non-noble metal electrocatalyst in fuel cells and metal–air batteries.
MnO2; MWCNTs-OH; Supercapacitor; Non-noble metal electrocatalyst
