- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
• Li[Li0.2Mn0.56Ni0.16Co0.08]O2 was synthesized by solid-state method.
• Effects of calcination temperature, time, and quenching methods were investigated.
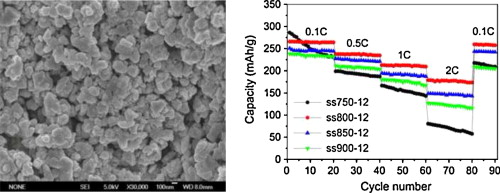
• Material synthesized at 800 °C for 12 h and quenched in air shows best electrochemical property.
Layered Li[Li0.2Mn0.56Ni0.16Co0.08]O2 cathode materials were synthesized via a solid-state reaction for Li-ion batteries, in which lithium hydroxide monohydrate, manganese dioxide, nickel monoxide, and cobalt monoxide were employed as metal precursors. To uncover the relationship between the structure and electrochemical properties of the materials, synthesis conditions such as calcination temperature and time as well as quenching methods were investigated. For the synthesized Li[Li0.2Mn0.56Ni0.16Co0.08]O2 materials, the metal components were found to be in the form of Mn4+, Ni2+, and Co3+, and their molar ratio was in good agreement with stoichiometric ratio of 0.56:0.16:0.08. Among them, the one synthesized at 800 °C for 12 h and subsequently quenched in air showed the best electrochemical performances, which had an initial discharge specific capacity and coulombic efficiency of 265.6 mAh/g and 84.0%, respectively, and when cycled at 0.5, 1, and 2 C, the corresponding discharge specific capacities were 237.3, 212.6, and 178.6 mAh/g, respectively. After recovered to 0.1 C rate, the discharge specific capacity became 259.5 mAh/g and the capacity loss was only 2.3% of the initial value at 0.1 C. This work suggests that the solid-state synthesis route is easy for preparing high performance Li[Li0.2Mn0.56Ni0.16Co0.08]O2 cathode materials for Li-ion batteries.