- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
• A commercial pigment composed of carbon nanospheres can be used for energy storage.
• The carbon nanospheres exhibit high capacitance in neutral aqueous electrolytes.
• Hydrous radius of the cation determines the measured capacitance.
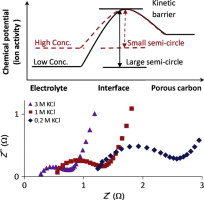
• Semicircles on AC impedance plots are due to ion transfer crossing the carbon/electrolyte interface.
• Capacitance changes with temperature disagree with classic theory of electrical double layer.
A commercial product of carbon nano-particles, Cabot MONACH 1300 pigment black (CMPB), was studied for basic structural information and electrochemical performance in neutral aqueous electrolytes, aiming at applications in supercapacitors. As confirmed by SEM and HRTEM, the CMPB had a hierarchical structure, containing basic 10 nm nano-spheres which combined into ca. 50 nm agglomerates which further aggregated into larger particles of micrometres. The capacitance of this commercial material was found to increase with decreasing the size of hydrous cation (Li+ → Na+ → K+), instead of the cation crystal radius (K+ → Na+ → Li+) when coupled with the same anion (Cl−). In electrolytes with the same cation concentration (K+), changing the anion from the larger dianion (SO42−) to the smaller monoanion (Cl−) also increased the capacitance at high potential scan rates (>50 mV/s). Increasing electrolyte concentration produced expected effect, including raising the electrode capacitance, but lowering the equivalent series resistance (ESR), charge transfer resistance (CTR), and the diffusion resistance. At higher temperatures, the CMPB exhibited slightly higher capacitance, which does not agree with the Gouy–Chapman theory on electric double layer (EDL). A hypothesis is proposed to account for the capacitance increase with temperature as a result of the CMPB opening up some micro-pores for more ions to access in response to the temperature increase.