- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
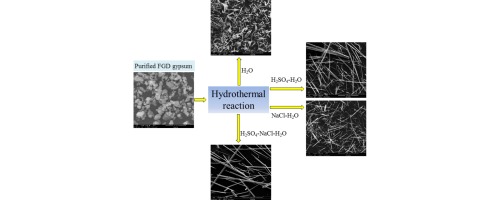
• Calcium sulfate whiskers (CSWs) were prepared from purified FGD gypsum in H2SO4–NaCl–H2O system.
• Crystallization of CSW could be controlled via the formulas of H2SO4 and NaCl.
• H2SO4 and NaCl have opposite effects on the solubility of FGD gypsum.
Little attention has thus far been paid to the potential effect of solution composition on the hydrothermal crystallization of calcium sulfate whiskers prepared from flue-gas desulfurization (FGD) gypsum. When purified FGD gypsum was used as raw material, the morphology and phase structure of the hydrothermal products grown in pure water, H2SO4–H2O, NaCl–H2O, and H2SO4–NaCl–H2O solutions as well as the solubility of purified FGD gypsum in these solutions were investigated. The results indicate that calcium sulfate whiskers grow favorably in the H2SO4–NaCl–H2O system. When prepared using 10–70 g NaCl/kg gypsum −0.01 M H2SO4–H2O at 130 °C for 60 min, the obtained calcium sulfate whiskers had diameters ranging from 3 to 5 μm and lengths from 200 to 600 μm, and their phase structure was calcium sulfate hemihydrate (HH). Opposing effects of sulfuric acid and sodium chloride on the solubility of the purified FGD gypsum were observed. With the co-presence of sulfuric acid and sodium chloride in the reaction solution, the concentrations of Ca2+ and SO42− can be kept relatively stable, which implies that the crystallization of the hydrothermal products can be controlled by changing the concentrations of sulfuric acid and sodium chloride.