- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
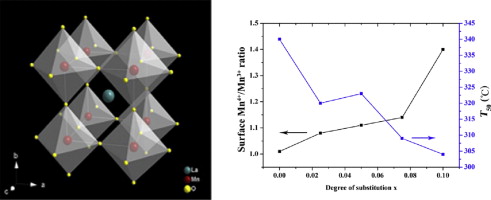
• Change in specific surface area has less impact on the catalytic activity of benzene oxidation.
• Mn4+/Mn3+ increased and Oads/Olatt decreased with Ce4+ substitution.
• Surface element species ratios correlated with catalytic activity.
Perovskite-type La1−xCexMnO3 (x = 0–10%) catalysts were prepared by flame spray pyrolysis and their activities during the catalytic oxidation of benzene were examined over the temperature range of 100–450 °C. The structural properties and reducibility of these materials were also characterized by X-ray diffraction (XRD), N2 adsorption/desorption, H2 temperature-programmed reduction (H2-TPR) and X-ray photoelectron spectroscopy (XPS). The incorporation of Ce was found to improve the benzene oxidation activity, and the perovskite in which x was 0.1 exhibited the highest activity. Phase composition and surface elemental analyses indicated that non-stoichiometric compounds were present. The incorporation of Ce had a negligible effect on the specific surface area of the perovskites and hence this factor has little impact on the catalytic activity. Introduction of Ce4+ resulted in modification of the chemical states of both B-site ions and oxygen species and facilitated the reducibility of the perovskite. The surface Mn4+/Mn3+ ratio was increased as a result of Ce4+ substitution, while a decrease in the surface-adsorbed O/lattice O (Oads/Olatt) ratio was observed. The relationship between the surface elemental ratios and catalytic activity was established to allow a better understanding of the process by which benzene is oxidized over perovskites.