- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
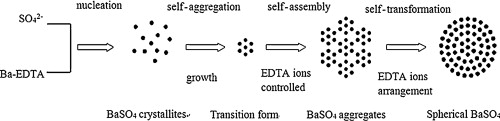
• Monodispersed spherical BaSO4 aggregates with an average size of 0.5 μm were fabricated at pH 7.
• The as-prepared particles were composed of interconnected nanoballs and self-assembled structures.
• Surface self-assembly and transformation mechanism was proposed which was directed by EDTA anions.
• The adsorption of OH− anions onto the surface of BaSO4 influenced the particle morphology.
Barium sulfate aggregates with an average size of 0.5 μm were synthesized at pH 7, directed by ethylenediaminetetraacetic acid (EDTA) anions. The particle morphology, chemical composition, and size distribution of the BaSO4 aggregates were characterized. The as-synthesized BaSO4 particles were spherical and comprised many interconnected nanoballs, of which the surface properties were affected by the EDTA anions. The adsorption of EDTA anions reversed the charge and weakened the surface polarity of BaSO4, instigating the formation of aggregates by a self-assembly and transformation process. The resulting BaSO4 particles at pH 9–10 were ellipsoidal and featured smooth surfaces. Based on the zeta potential of BaSO4, variations in the morphology induced by changes in pH were closely related to the adsorption of mono- and multi-valent anions onto the electrical double layer of BaSO4.