- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
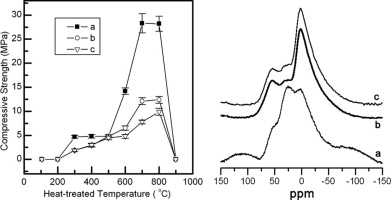
• Powder prepared by sol–gel method had higher alkali-activation reactivity.
• The powders were investigated by 27Al and 29Si MAS NMR.
• The Al(V) contents of the powders were in agreement with their alkali-activation reactivities.
• Si was replaced by Al at secondary coordination sites during calcination of the powders.
Pure Al2O3–2SiO2 powders were prepared by sol–gel and coprecipitation methods, and their alkali-activation reactivities were compared. The alkali-activation reactivity of the powder prepared by the sol–gel method was higher than that of the powder prepared by the coprecipitation method. The powders were investigated by 27Al and 29Si magic-angle spinning nuclear magnetic resonance spectroscopy (MAS NMR) to understand the relationship between their structure and alkali-activation reactivity. The 27Al MAS NMR data showed that the five-coordinate Al content of the powder prepared by the sol–gel method was higher than that of the powder prepared by coprecipitation. The higher content of five-coordinate Al corresponded to higher alkali-activation reactivity. The 29Si MAS NMR data showed that for the powder prepared by the sol–gel method, silicon was replaced by aluminum at secondary coordination sites of the central Si atoms during calcination. However, for the powder prepared by single-batch coprecipitation, the main change was from a low degree of polycondensation to a high degree of polycondensation.