- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
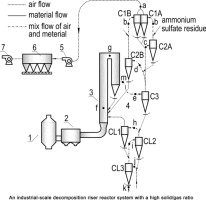
• Ammonium sulfate residue was decomposed in suspension state with a high solid/gas ratio.
• An industrial-scale reactor system (production line) was developed to produce active CaO via decomposition.
• Optimum operating conditions for the decomposition were determined.
• The product had a porous structure and a large specific surface ensuring high activity.
Ammonium sulfate residue is a particulate solid and is produced during the manufacture of ammonium sulfate fertilizer. The residue used in this study contained a large portion of calcium carbonate, from which active lime (CaO) was recovered via thermal decomposition. We used a purpose-built device to decompose the residue in a semi-suspension state. We found that CaO had the highest activity when residue was decomposed at 850–900 °C. Our experiments indicated that ammonium sulfate residue should be decomposed in a suspension state to produce active CaO. Based on our laboratory test findings, an industrial-scale production line with a high solid/gas ratio in a suspension state was devised. The optimal operating conditions for the decomposition of the ammonium sulfate residue to produce high quality CaO were also investigated. We found that the CaCO3 decomposition rate was high and the CaO product was highly active, averaging 170 s by the citric acid method. Morphology measurements showed that the CaO product had a porous structure and a large specific surface ensuring high activity.