- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
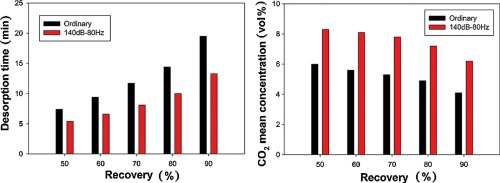
• Higher desorption times gave higher CO2 recoveries and lower CO2 purities.
• The desorption rate was enhanced under sound assisted conditions.
• The application of the sound yielded a remarkable enrichment of the recovered CO2.
• Regeneration was very stable under sound assisted fluidization conditions.
Adsorption using solid sorbents has the potential to complement or replace current absorption technology, because of its low energy requirements. Among the commercially available adsorbent materials, attention is focused on activated carbons because they are easily regenerable by reason of their low heat of adsorption. These sorbents are generally available in the form of fine powders. Sound-assisted fluidization can process large amounts of fine powders, promoting and enhancing CO2 capture on fine sorbents, because it maximizes gas–solid contact. Temperature swing adsorption (TSA), consisting of inducing sorbent regeneration and CO2 recovery by appropriate temperature increase and gas purge, is one of the most promising techniques. This study investigates the CO2 desorption process by TSA in a sound-assisted fluidized bed of fine activated carbon. Desorption tests were performed under ordinary and sound-assisted fluidization conditions to assess the capability of sound to promote and enhance the desorption efficiency in terms of CO2 recovery, CO2 purity, and desorption time. The results show that the application of sound results in higher desorption rates, CO2 recovery and purity. Regular and stable desorption profiles can be obtained under sound-assisted fluidization conditions. This stability makes it possible to successfully realize a cyclic adsorption/desorption process.
Temperature swing adsorption; Sound-assisted fluidization; CO2 capture; Activated carbons; Fine powders
