- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
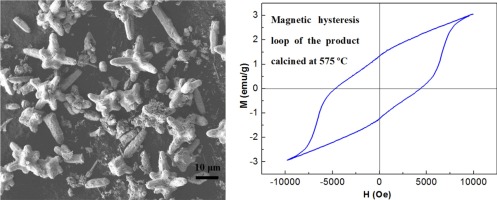
• Star-shaped Sr3Fe2(OH)12 assemblies were synthesized via a mild hydrothermal route.
• Impurities could be mitigated by increasing the molar ratio of Sr/Fe.
• Thermal decomposition and magnetic properties were investigated.
Pure phase star-shaped hydrogarnet Sr3Fe2(OH)12 assemblies were synthesized by a mild hydrothermal method (210 °C, 12 h), and the effects of the preparation conditions on the phase composition of the product were investigated. It was found that the impurity phases could be decreased or eliminated by increasing the molar ratio of Sr2+ to Fe3+, and that high temperatures favored the formation of Sr3Fe2(OH)12 and reduced the concentration of CO32–-containing byproducts. The thermal decomposition of the star-shaped Sr3Fe2(OH)12 assemblies was examined, and the results showed that the dehydration process at higher temperatures is accompanied by the formation of SrFeO3–δ. Above 655 °C, a solid state reaction between the SrFeO3–δ and Sr(OH)2 or SrCO3 results in the formation of Sr4Fe3O10–δ.The magnetic properties of the as-synthesized Sr3Fe2(OH)12 and of samples calcined at different temperatures were assessed. A sample calcined at 575 °C exhibited greatly enhanced ferromagnetic properties, with a remanent magnetization of 1.28 emu/g and a coercivity of 4522.1 Oe at room temperature.