- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
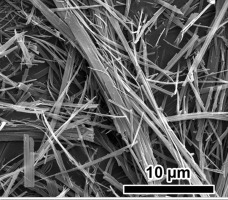
• 5Mg(OH)2·MgSO4·3H2O (513MOS) whiskers were synthesized by an atmospheric pressure reflux method.
• Long reaction time and high MgSO4 concentration were necessary under gentle reaction conditions.
• The growth of 513MOS whiskers is a relatively slow liquid phase deposition process.
• Porous MgO whiskers were derived via 1,020 °C calcination from 513MOS whiskers.
We have developed a one-step process for the synthesis of basic magnesium sulfate (5Mg(OH)2·MgSO4·3H2O, abbreviated as 513MOS) whiskers from MgSO4·7H2O and MgO by refluxing at atmospheric pressure. The process shows potential for the low-cost mass production of controlled-structure whiskers. Their 0.3–1.0 μm diameter and 40–80 μm length correspond to an aspect ratio of 40–260. The 513MOS whisker morphology is related closely to MgSO4 concentration and reflux time. The optimized MgSO4 concentration is 1.2–1.5 mol/L with a 25–30 h reflux time. X-ray diffractometry revealed that the b-axis is the predominant growth direction of the whiskers. Their growth mechanism is by the relatively slow liquid-phase deposition of Mg2+, OH–, and SO42–. The long reaction time and high MgSO4 concentration are conducive to the formation of 513MOS whiskers under gentle reaction conditions. Porous MgO whiskers with a fibrous structure were obtained after calcination of the 513MOS whiskers at 1020 °C.