- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
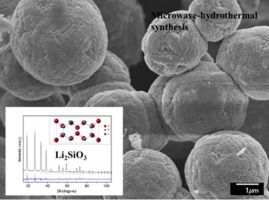
• Nanocrystalline Li2SiO3 powders were synthesized via microwave-assisted hydrothermal method.
• Pure Li2SiO3 phase was obtained using short reaction time at low temperature.
• Li2SiO3 particles exhibited hollow sphere morphologies and high specific surface area.
• Water vapor and CO2 capture properties were evaluated by thermogravimetric analysis.
• Exhibited properties suggested potential applications of Li2SiO3 as catalyst and CO2 sorbents.
A series of lithium metasilicate (Li2SiO3) powder materials has been successfully synthesized by the microwave-assisted hydrothermal route using lithium hydroxide and tetraethyl-orthosilicate-derived sol precursors. Ceramic powders were obtained under hydrothermal conditions of autogenous pressure in the presence of a nonionic surfactant. The production of pure and well-crystallized Li2SiO3 using very short reaction times at low temperatures was shown by X-ray diffraction, scanning electron microscopy, and N2 adsorption-desorption analyses. Synthesized Li2SiO3 particles were nanocrystalline and exhibited different morphologies and specific surface areas depending on the synthesis conditions. Additionally, the capability of selected Li2SiO3 samples to absorb H2O and CO2 was evaluated via thermogravimetric analyses by varying the temperature, carrier gas, and water vapor concentration. Li2SiO3 particles exhibited interesting textural and morphological characteristics that make them suitable for use as a CO2 absorbent and which suggest that they also have the potential to be used in other applications.