- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
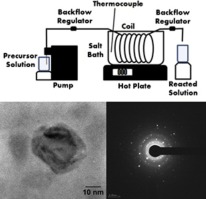
• A continuous flow thermal decomposition method for producing iron oxide nanoparticles was studied.
• Iron oxide nanoparticles produced in the continuous flow reactor were crystalline.
• Iron oleate/oleic acid molar ratio was the key factor in deciding particle size in this process.
• A continuous flow reaction mechanism was proposed.
Iron oxide nanoparticles have become of great interest in the medical field for their potential uses in areas such as biomagnetic imaging and hypothermia cancer treatment. Traditionally, particles for these applications are produced through batch-based methodologies. Herein, we demonstrate an alternative continuous flow production method for the synthesis of Fe3O4 iron oxide nanoparticles. Advantages of continuous flow over the batch method include consistent formation of uniformly spherical particles, thorough mixing of reactants, and capacity for high-volume particle production. In this study, a continuous flow reaction mechanism was proposed in which stoichiometric control of reactants had the potential to control final particle size. The project was conducted under the supposition that the iron oleate/ligand ratio in the precursor was the greatest size control factor, with a higher ratio resulting in smaller particles. The resulting particles produced by this continuous method were characterized by high-resolution transmission electron microscopy, X-ray diffraction, and magnetometry.