- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
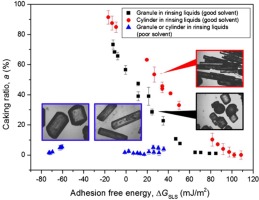
• Particles shape and solubility were studied for caking mechanism.
• The research based on theories of adhesion free energy and crystal bridge.
• Relationship between caking ratio and adhesion free energy was studied.
• Caking could be inhibited by screening rinsing liquids, and optimizing particle shape and size.
We investigated the influence of particle shape and solubility on the caking behavior of trisodium phosphate by considering the adhesion free energy and crystal bridge theory. Caking of trisodium phosphate during the drying process under static conditions is a two-step process: adhesion followed by crystal bridge formation between particles. The adhesion free energy plays an important role in adhesion. Trisodium phosphate particles cannot adhere to each other and cake when the adhesion free energy is greater than a critical value, which varies with particle shape. Compared with granular particles, cylindrical particles have larger contact area between particles, which results in more crystal bridges forming and a higher caking ratio. Thus, the critical value is about 100 mJ/m2 for cylindrical particles, but 60 mJ/m2 for granular particles at 25 °C. Concerning the solubility, when particles are similar shapes and soluble in the rinsing liquid, the caking ratio has a linear relationship with adhesion free energy. However, if the particles are insoluble in the rinsing liquid, caking can be completely prevented regardless of adhesion free energy because no crystal bridges form during the growth process. Hence, caking of trisodium phosphate particles could be inhibited by screening rinsing liquids, and optimizing the particle shape and size distribution.