- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
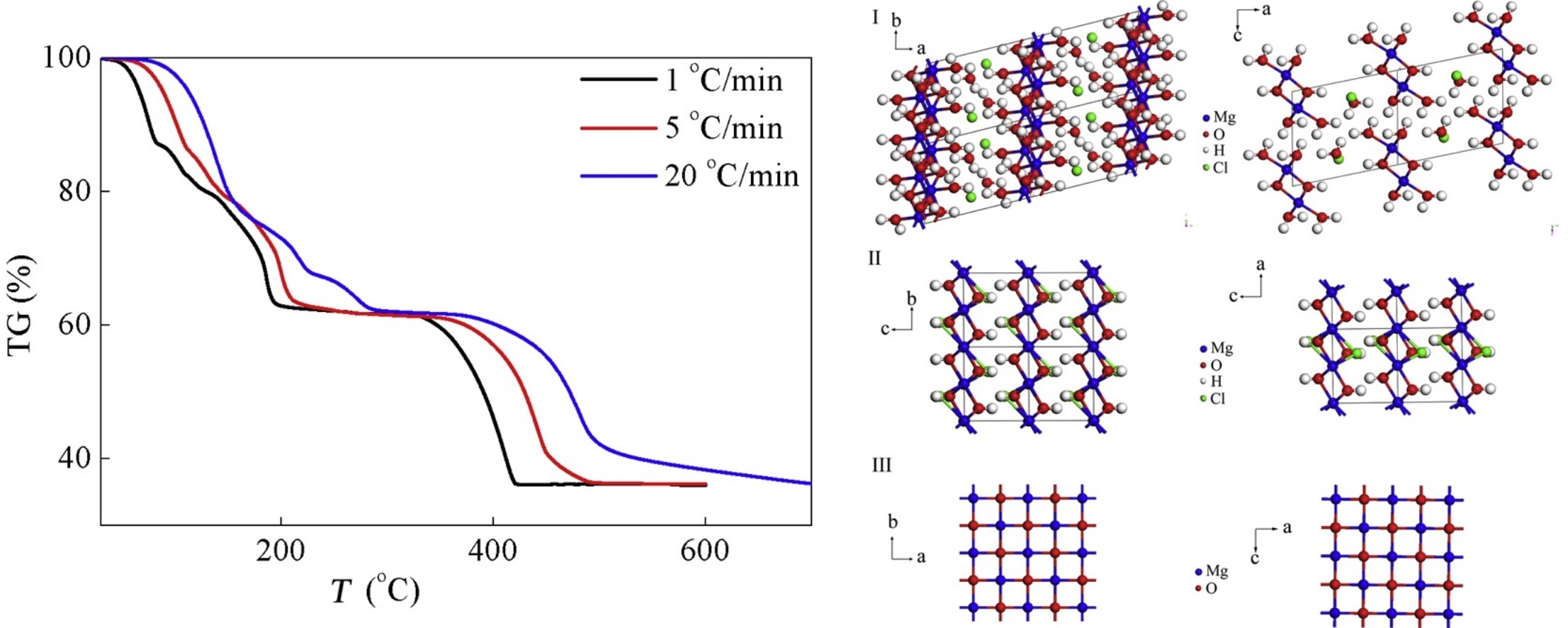
• Effect of heating rate on thermal decomposition of 318MHCH was investigated.
• Physically adsorbed and crystalline water were separated from DTG curve by curve fitting method.
• Immediate collapse of 1D nanowires was identified and discussed upon heating up to 420 °C.
• Double chains model was proposed to explain the immediate collapse.
The thermal decomposition of 3Mg(OH)2·MgCl2·8H2O (318MHCH) nanowires synthesized from agglomerated Mg(OH)2 microspheres was investigated. The influence of heating rate and temperature on the composition and morphology of the products was investigated. Thermogravimetric-differential scanning calorimetry, field-emission scanning electron microscopy, high-resolution transmission electron microscopy, and X-ray diffraction showed that increasing the heating rate from 1 to 20 °C/min promoted the escape of crystalline water from the 318MHCH nanowires. 318MHCH nanowires were dehydrated stepwise to 310MHCH porous nanowires from room temperature to 320 °C, and then to MgO cubic nanoparticles from 420 to 700 °C. The nanowires retained their one-dimensional morphology, until the phase changed to MgO. The immediate collapse of the one-dimensional structure was attributed to the presence of Mg–O/Cl chains.