- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
Yan Feng a b, Jinding Pan a b, Hui Liu a c, Jun Yang a c *
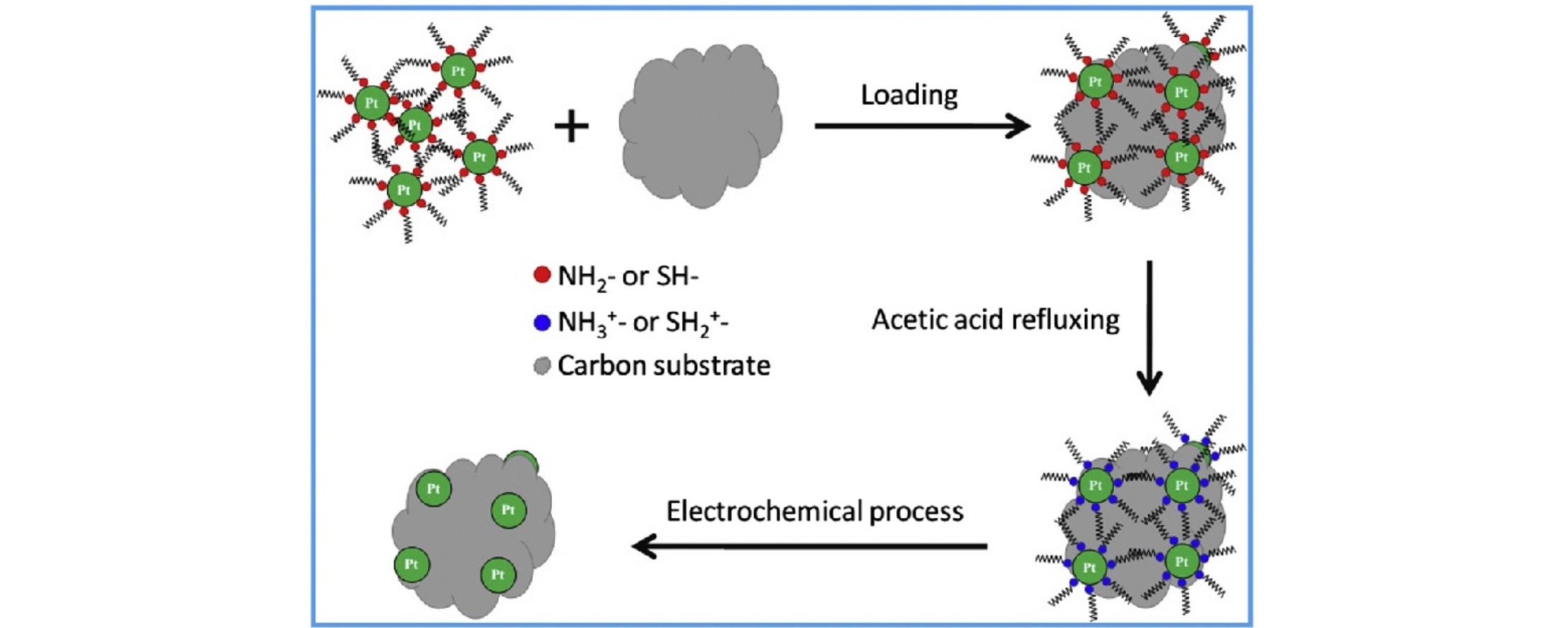
• Pt nanoparticles capped with amine- or thiol-based surfactants were loaded on carbon substrates.
• The amine- or thiol-based surfactants were protonated by refluxing in acetic acid.
• The protonated surfactants were removed from Pt particle surface by an electrochemical process.
• The activated Pt particles exhibited higher activity for methanol oxidation and oxygen reduction.
Surfactant removal from the surface of platinum-based nanoparticles prepared using solution-based methods is a prerequisite to realize their high catalytic performance for electrochemical reactions. Herein, we report an effective approach combining acetic acid refluxing with an electrochemical process for the removal of amine- or thiol-based capping agents from the surface of supported-platinum nanoparticles. This strategy involves surfactant protonation by refluxing the supported-platinum particles in acetic acid followed by surfactant removal by subsequent electrochemical treatment at high potential. We demonstrate that this combined activation process is essential to enhance platinum particle performance in catalyzing direct methanol fuel cell reactions, including methanol oxidation and oxygen reduction reactions. The studies in this work show promise in electrocatalysis applications of solution-based materials synthesis.