- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
• LiMnPO4/C composites were synthesized by microwave assisted polyol method in a short time.
• Micromorphology and particle size of the prepared samples were adjusted by using surfactants.
• LiMnPO4/C particles prepared with PVPk30 had a flaky form coated with a uniform carbon layer.
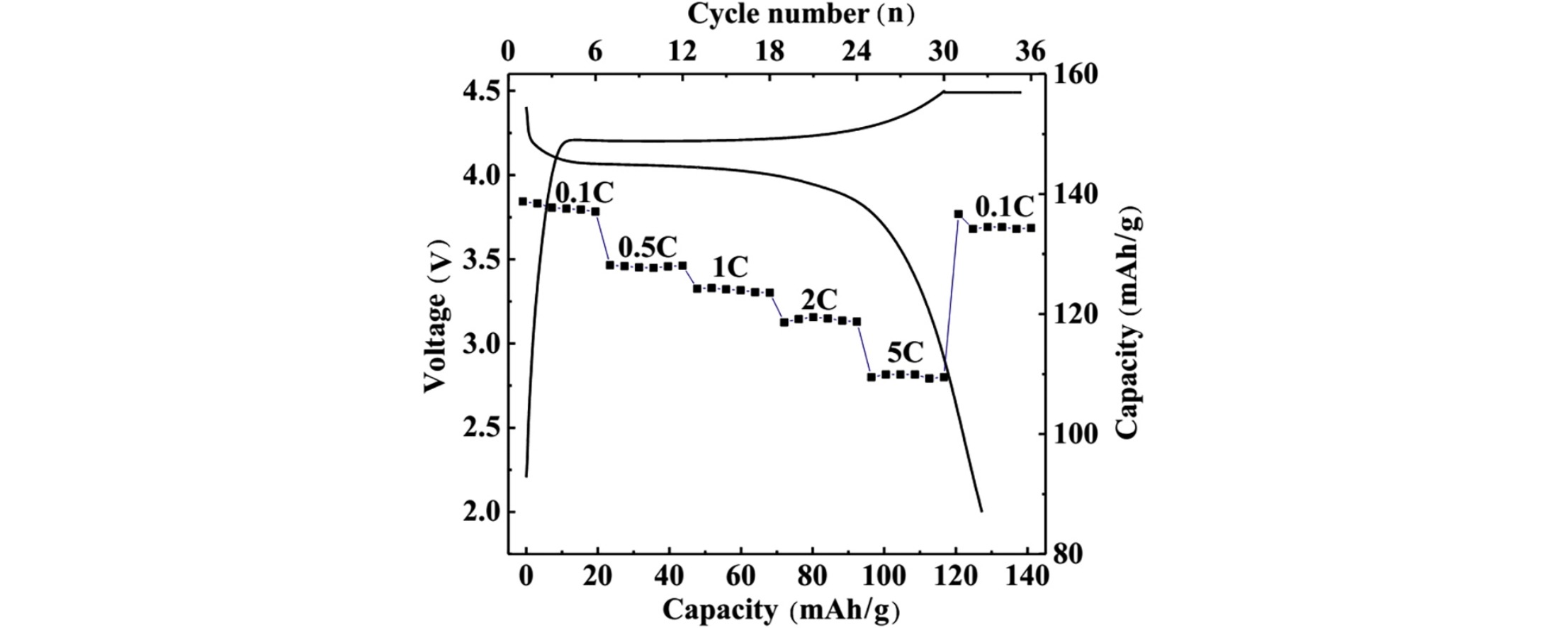
• Flaky LiMnPO4/C composites exhibited a good rate performance and cycle stability.
We synthesized LiMnPO4/C with an ordered olivine structure by using a microwave-assisted polyol process in 2:15 (v/v) water–diethylene glycol mixed solvents at 130 °C for 30 min. We also studied how three surfactants—hexadecyltrimethylammonium bromide, polyvinylpyrrolidone k30 (PVPk30), and polyvinylpyrrolidone k90 (PVPk90)—affected the structure, morphology, and performance of the prepared samples, characterizing them by using X-ray diffraction, scanning electron microscopy, transmission electron microscopy, charge/discharge tests, and electrochemical impedance spectroscopy. All the samples prepared with or without surfactant had orthorhombic structures with the Pnmb space group. Surfactant molecules may have acted as crystal-face inhibitors to adjust the oriented growth, morphology, and particle size of LiMnPO4. The microwave effects could accelerate the reaction and nucleation rates of LiMnPO4 at a lower reaction temperature. The LiMnPO4/C sample prepared with PVPk30 exhibited a flaky structure coated with a carbon layer (∼2 nm thick), and it delivered a discharge capacity of 126 mAh/g with a capacity retention ratio of ∼99.9% after 50 cycles at 1 C. Even at 5 C, this sample still had a high discharge capacity of 110 mAh/g, demonstrating good rate performance and cycle performance. The improved performance of LiMnPO4 likely came from its nanoflake structure and the thin carbon layer coating its LiMnPO4 particles. Compared with the conventional polyol method, the microwave-assisted polyol method had a much lower reaction time.