- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
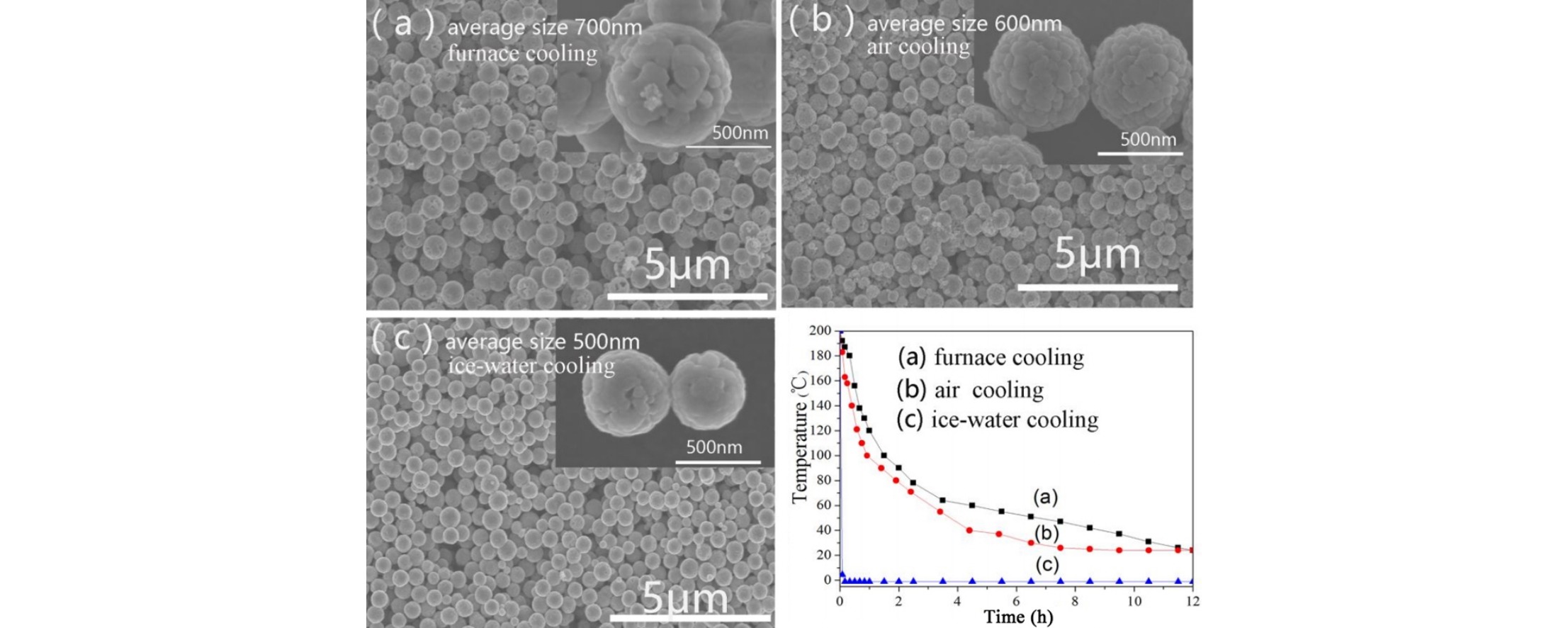
• Effect of cooling rate on the growth of Fe3O4 nanospheres was investigated.
• Higher cooling rate improved crystallinity and magnetic properties of the nanospheres.
• Growth mechanism of the hollow magnetite oxide nanospheres was proposed.
• Correlation between the structure and magnetic property of the nanospheres was discussed.
The controlled synthesis of hollow magnetite (Fe3O4) nanospheres of varying sizes and structures was successfully obtained via a facile solvothermal process and varying cooling processes. The Fe3O4 nanospheres were characterized by X-ray diffraction, transmission electron microscopy, scanning electron microscopy, and superconducting quantum interference device magnetometry. The diameters of the as-synthesized nanospheres were controlled at around 500–700 nm by simply changing the cooling rate, which had an obvious influence on the morphology and magnetic properties of these Fe3O4 nanospheres. While a low cooling rate triggered the formation and extension of the cracks present in the Fe3O4 nanospheres, a sudden drop of temperature tended to favor multi-site nucleation of the crystals as well as the formation of compact and smooth hollow nanospheres with superior crystallinity and high saturation magnetization. The growth mechanism of hollow magnetite oxide nanospheres was proposed and the correlation between the structure and the magnetic properties of the hollow nanospheres was discussed, which promises the potential of the hollow nanospheres in various applications such as drug delivery and cell separation.