- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
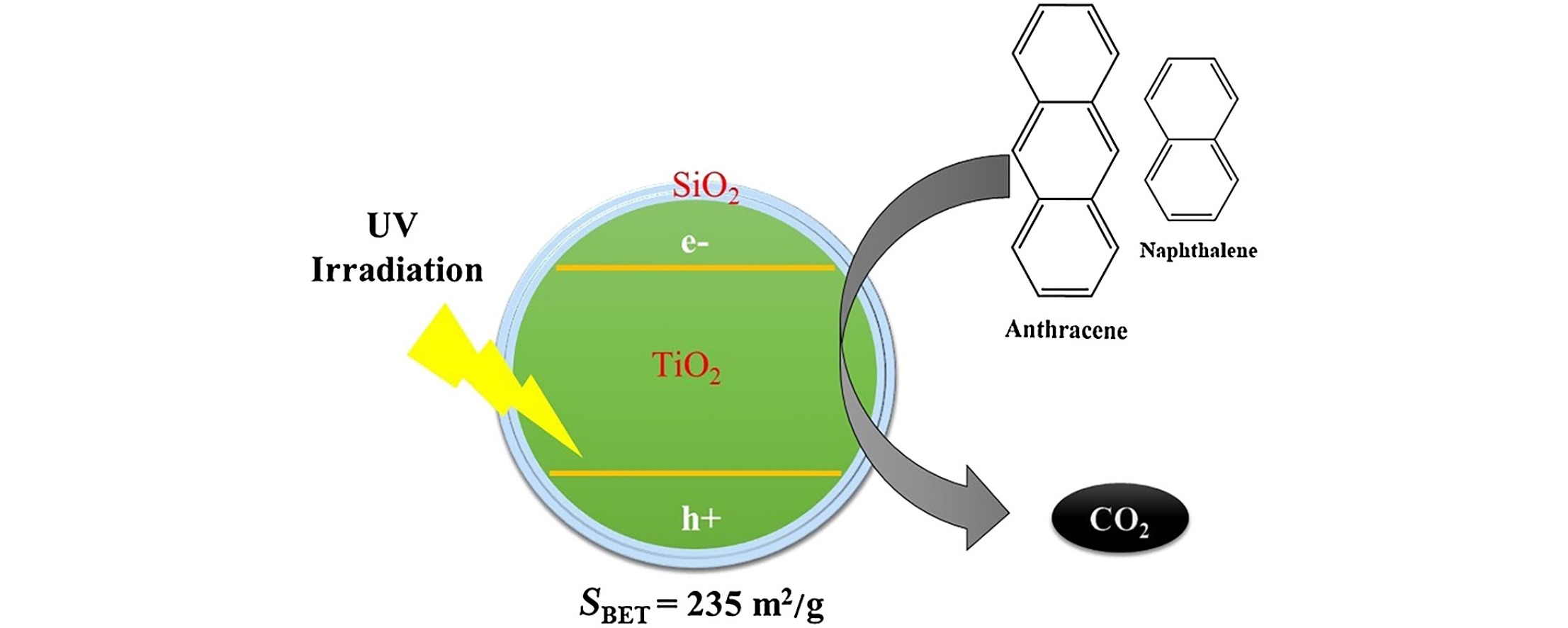
• SiO2 coated TiO2 nanoparticles were synthesized with different silica shell thicknesses.
• More adsorption of naphthalene/anthracene was found due to higher surface area of SiO2 coated TiO2.
• Silica coated TiO2 showed higher activity for degradation of naphthalene and anthracene to CO2.
• Complete mineralization of naphthalene and anthracene had been obtained in 240 min.
Our current efforts reveal the preparation of SiO2@TiO2 nanocomposites having different thicknesses of silica shell and the relationship to photocatalytic activity (PCA) for the photo-oxidation of naphthalene and anthracene. The presence of SiO2 coating over TiO2 surface was demonstrated by FT-IR analysis, with peaks corresponding to Sisingle bondOsingle bondSi (1081 cm−1) and Sisingle bondOsingle bondTi (950 cm−1) bonds observed. High-resolution transmission electron microscopy analysis confirmed the presence of SiO2 in the as-prepared nanocomposites and the amount of Si, Ti, and O was determined by energy dispersive X-ray spectroscopy analysis. Increasing the SiO2 shell thickness increases the surface area of the nanocomposites (69–235 m2/g), which enhances naphthalene/anthracene adsorption. However, the observed PCA trend presents an inverse correlation to the adsorption studies, where the as-prepared samples possessing the highest surface areas exhibited the least PCA, while catalysts having lower surface areas (among silica coated samples) displayed the highest PCA in the degradation of naphthalene and anthracene to CO2. Despite complete degradation of naphthalene and anthracene, incomplete mineralization occurred, ascribed to the formation of various intermediates, identified by GC–MS analysis.