- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
• High gravity and hydrothermal technologies were used to prepare nitrogen-doped graphene.
• Graphene oxide reduction and nitrogen doping were accomplished with hydrothermal method.
• The nitrogen-doped graphene had better ORR catalytic properties.
• The mechanisms of nitrogen doping and graphene oxide reduction were proposed.
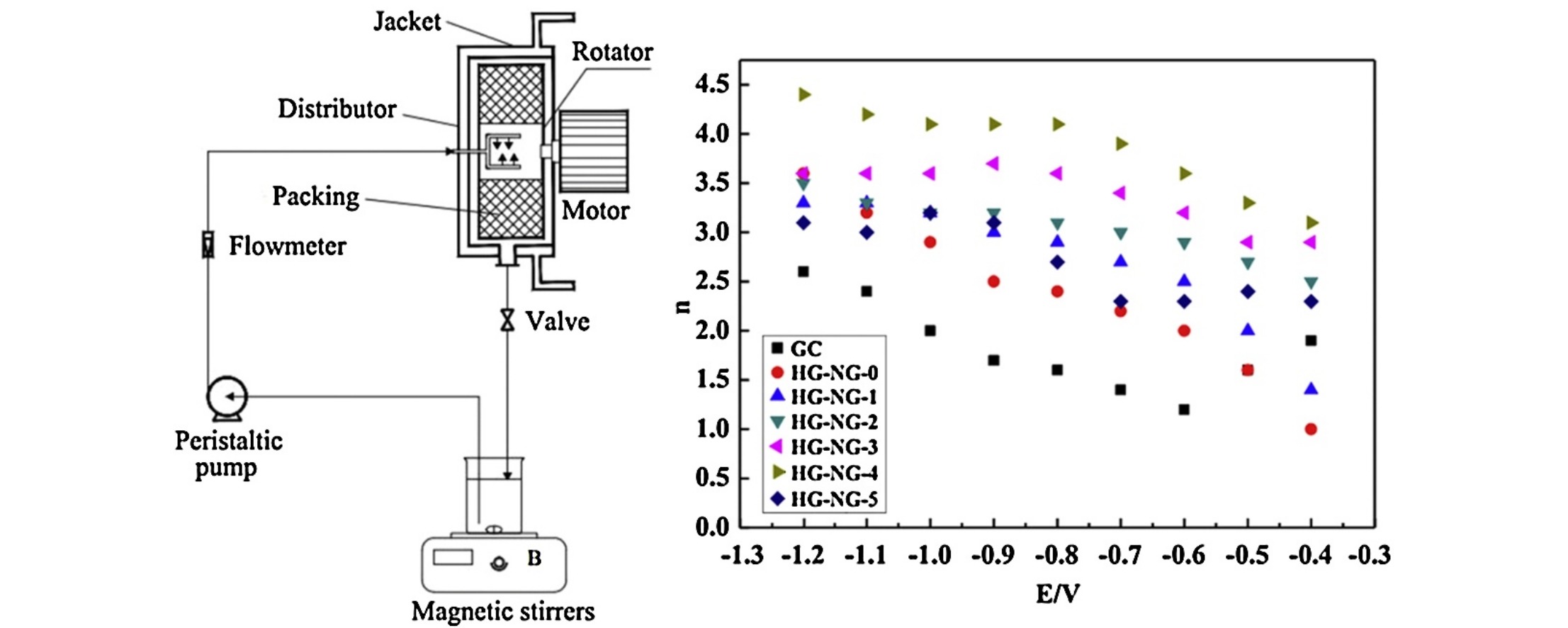
Electrochemical oxygen reduction is key to many clean and sustainable energy technologies, including proton exchange membrane fuel cells and metal–air batteries. However, the high activation barriers in the oxygen reduction reaction often make it the bottleneck of energy conversion processes; thus, high-performance oxygen reduction electrocatalysts are desired. At present, the best commercially available oxygen reduction catalyst is based on the precious metal Pt. However, it suffers from resource scarcity and unsatisfactory operational stability, hindering its widespread and large-scale application in clean and sustainable technologies. Nitrogen-doped graphene has excellent electrocatalytic properties for oxygen reduction. In this paper, a scalable method to prepare nitrogen-doped graphene with high quality was introduced, in which the graphene oxide prepared by high-gravity technology and urea was reacted under hydrothermal conditions. Accompanying the hydrothermal reaction, graphene oxide reduction and nitrogen doping were accomplished at the same time. The effect of the content of nitrogen on the performance of nitrogen-doped graphene was investigated. When the mass ratio (graphene oxide/urea) was 1:400, the nitrogen-doped graphene had the best oxygen reduction performance. Compared with the undoped samples, the initial reduction voltage of the nitrogen-doped samples distinctly shifted 45 mV to the right. When the voltage was −1.0 V, the electron transfer number was 4.1, indicating good oxygen reduction activity. The preparation method is feasible, simple, and can be easily scaled up.