- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
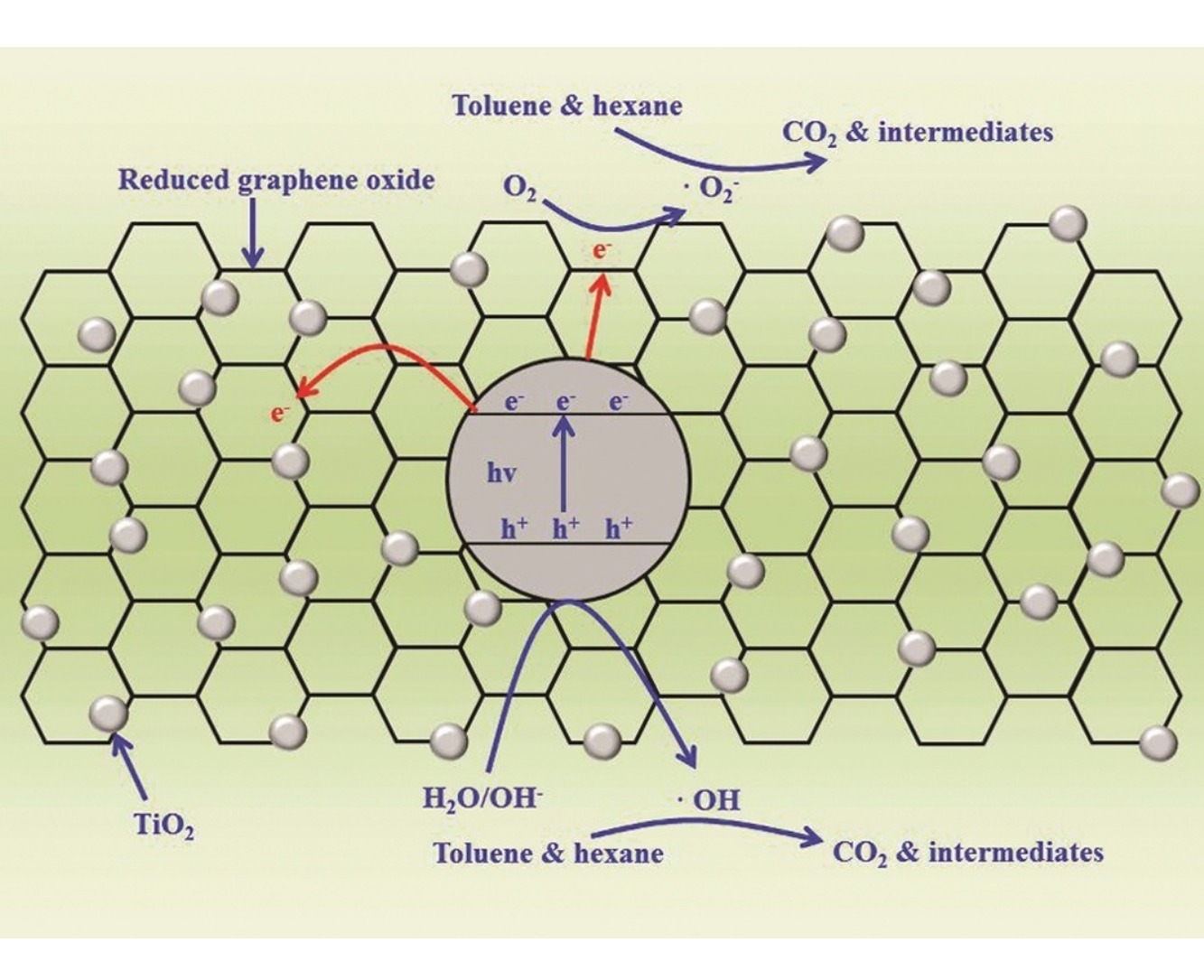
• Graphene–TiO2 composites were prepared using various hydrothermal conditions.
• The synthesized composites showed higher activity with daylight exposure.
• An optimal hydrothermal reaction time for the synthesis of composites existed.
• Violet LEDs were energy-efficient for degradation of gas pollutants over composites.
• Mass transfer, rather than surface reaction, could limit photocatalytic reaction rate.
Two-dimensional reduced graphene oxide–titania (RGO–TiO2) composites were prepared using a single-step hydrothermal method under various hydrothermal reaction conditions. The morphological and surface characteristics of the RGO–TiO2 composites and reference materials were determined. The RGO–TiO2 composites showed photocatalytic activity for the decomposition of two target pollutants that was superior to both pure TiO2 and RGO under fluorescent daylight lamp illumination. The photocatalytic activity of the RGO–TiO2 composite increased as the hydrothermal treatment time increased from 1 to 24 h, but then it decreased as the time increased to 36 h, which indicated the presence of an optimal treatment time. RGO–TiO2 composites activated by violet light-emitting diodes (LEDs) displayed lower decomposition efficiency than those activated by a daylight lamp, likely because of the lower light intensity of violet LEDs (0.2 mW/cm2) when compared with that of the daylight lamp (1.4 mW/cm2). However, the photocatalytic decomposition of the target pollutants using the RGO–TiO2 composite was more energy-efficient using the violet LEDs. The photocatalytic reaction rates increased as the residence time decreased, whereas the reverse was true for the decomposition efficiency.