- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
• Two-dimensional lead oxide sheets were synthesized by discharge in liquid nitrogen.
• The sheets are single crystals and belong to a new lead oxide phase.
• The sheets grow by gas phase condensation and not by a surface process.
• After sufficiently long discharge treatment, nanosticks were obtained by electrode erosion.
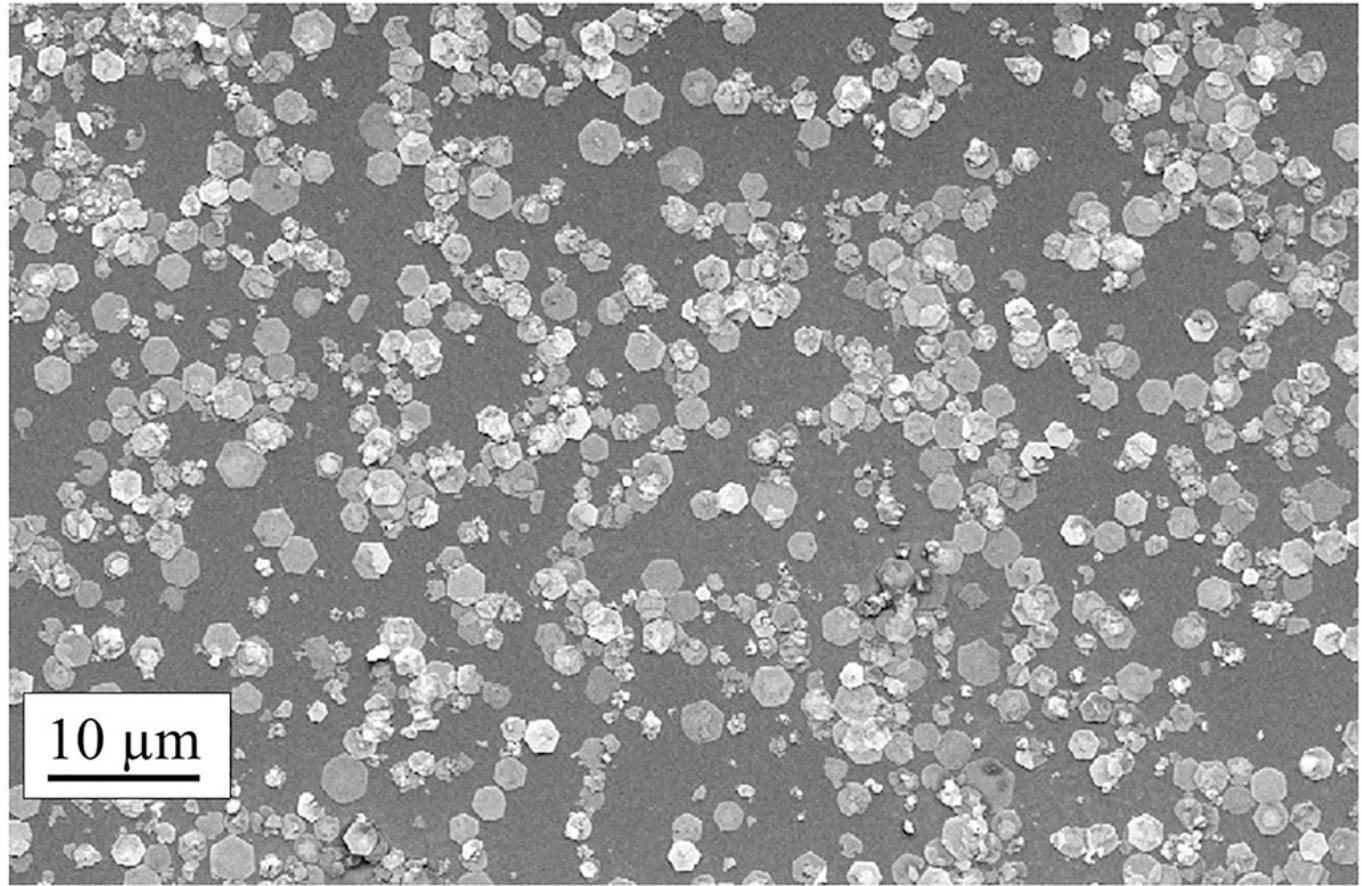
A simple method to synthesise hexagonal lead sheets, which belong to the class of two-dimensional materials, is proposed. These objects are collected on a substrate located under two lead electrodes, between which nanosecond-pulsed spark discharges are ignited in liquid nitrogen. The hexagonal sheets are single crystals produced by gas phase condensation. Once nitrogen completely evaporates, the sheets change to PbO2 by oxidation in air. The oxidation process induces stress that pleats the uppermost sheets or open cracks at the centre. The thickness of the individual objects typically varies from 4 to 20 nm. When the number of discharges is more than about 2000, in addition to sheets, two types of PbO2 sticks start to form: bundles composed of nanosticks (length 5 μm and diameter 50 nm) and isolated sticks (length 20 μm and diameter 500 nm). These new nanostructures mainly form because of the way the discharge erodes the lead electrodes. Initially, anisotropic erosion driven by the orientation of the crystallographic planes of the lead crystals produces octahedra and nanosticks, and the nanosticks grow on the electrode surfaces as discharge proceeds. After about 2000 discharges, the nanosticks are sufficiently long that they can be easily broken, probably by mechanical stress, and they fall onto the underlying substrate.