- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
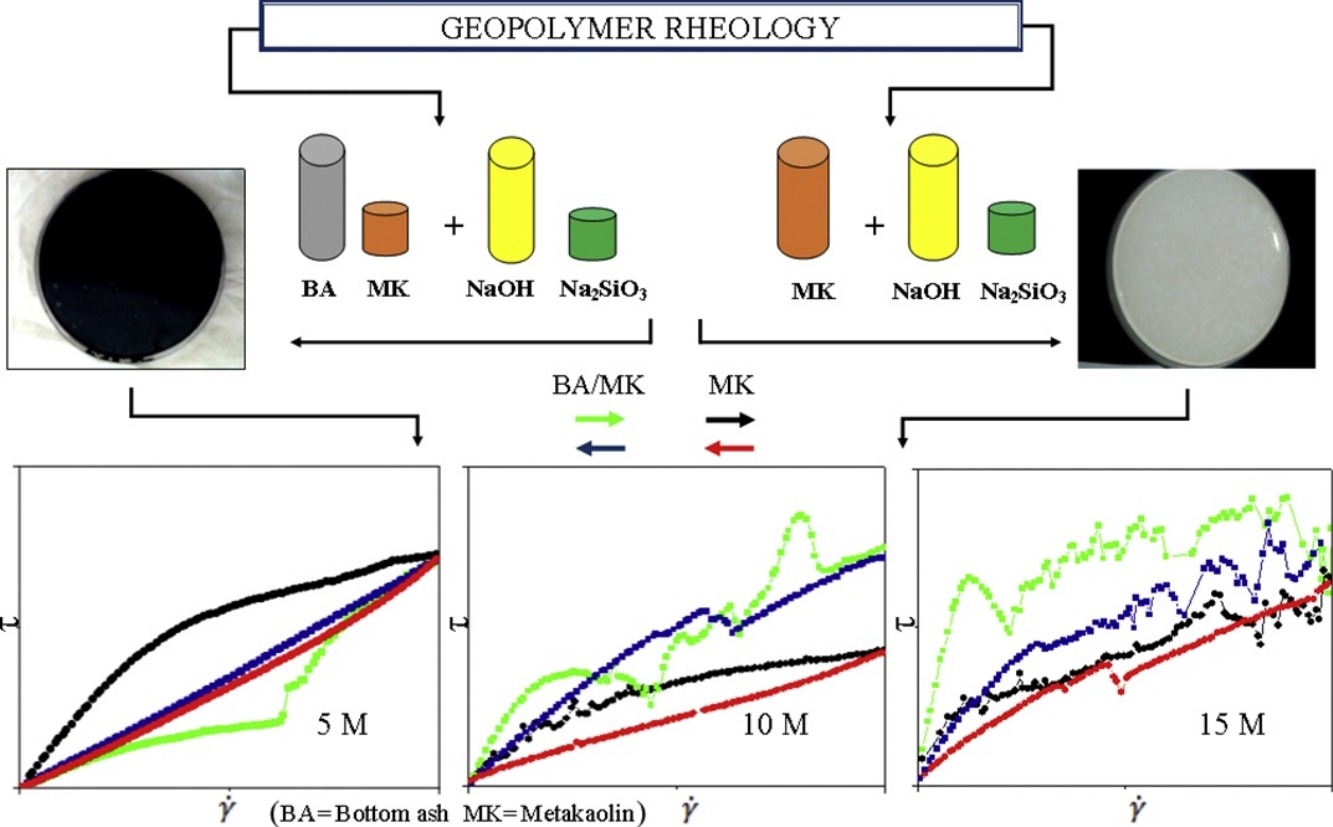
• Bottom ash/metakaolin and neat metakaolin were used to prepare geopolymer with NaOH as activator.
• Geopolymer formation was studied based on XRD patterns and FTIR spectra.
• Rheological behavior in geopolymer pastes was evaluated through viscosity and yield stress study.
• Activator molarity and water proportion affected the geopolymer system mostly.
The rheological behavior of microparticulate structures of geopolymers was studied. The materials were developed from alternative sources of aluminosilicate and were activated in different concentrations using 5, 10, and 15 M sodium hydroxide, and sodium silicate, which were added in a 2:1 ratio (by volume). The solid constituents used were bottom ash/metakaolin and neat metakaolin in a 2:1 blend. The rheological characteristics were determined after 5 min of homogenization and a subsequent curing of the pastes at room temperature for 28 days. The materials presented amorphous characteristics, which confirmed the formation of geopolymers. A thixotropic behavior was observed for all formulations, except for bottom ash/metakaolin activated at 5 M, which resulted in geopolymers with a lower intensity compared with those activated at 10 and 15 M. High activator concentrations exhibited a proportional relationship for geopolymer ring growth, which resulted in a reduced structure mobility and, consequently, an increased viscosity and yield stress. The effects are more evident in the metakaolin samples, and are associated with the water content that is required for reaction. Samples that were activated with 10 M sodium hydroxide presented a favorable workability and particle packing, in terms of the rheological and structural aspects.