- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
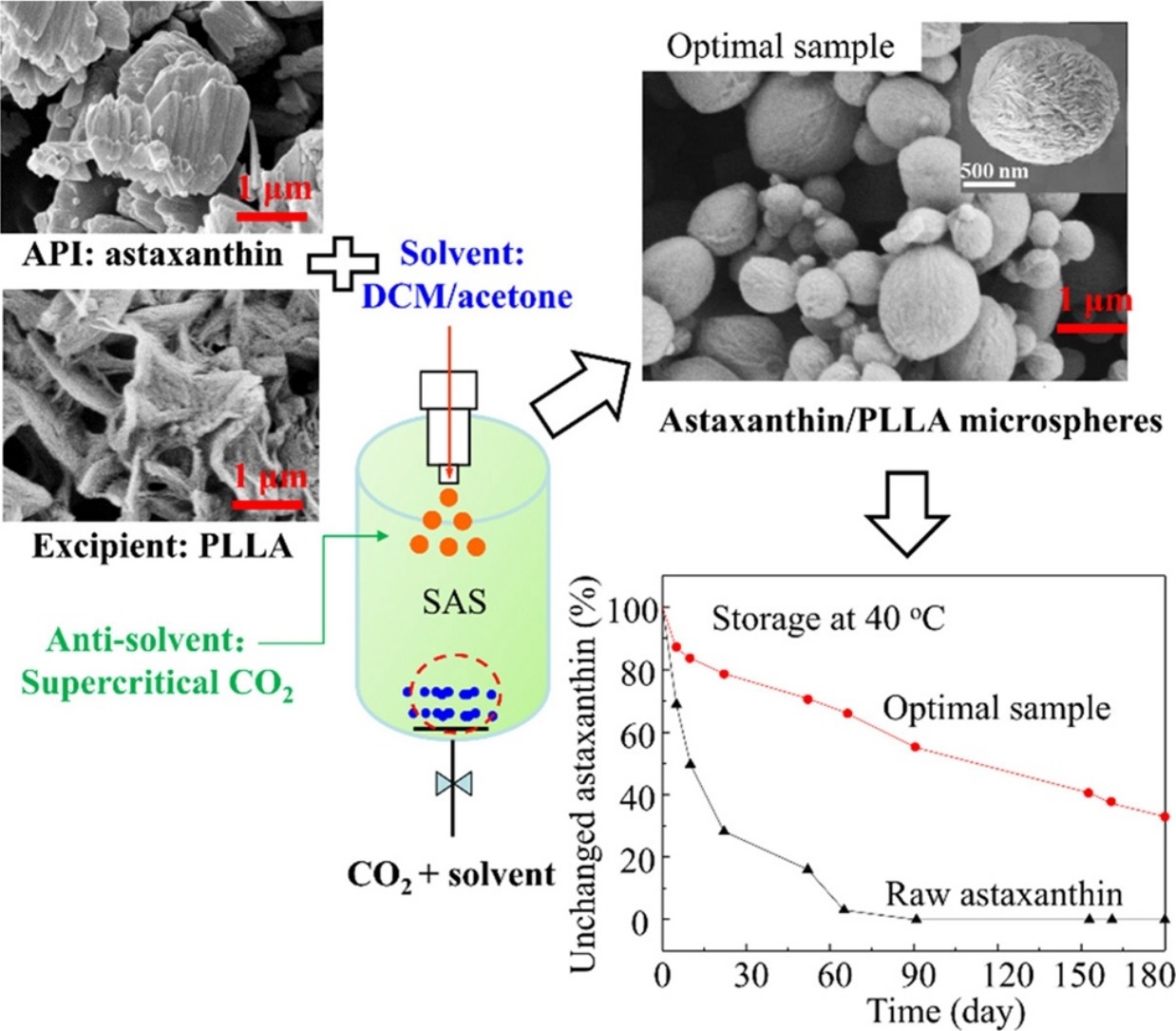
• Astaxanthin was successfully encapsulated into PLLA matrix via a SAS process.
• Key process variables affecting particle microstructures were investigated.
• The optimal particles were uniform microspheres with 91.5% encapsulation efficiency.
• The storage stability of astaxanthin could be greatly enhanced when loaded by PLLA.
To improve the physicochemical properties of astaxanthin, it was encapsulated in poly (l-lactic acid) (PLLA) using a supercritical anti-solvent (SAS) process with dichloromethane/acetone mixture as the solvent, and supercritical CO2 as the anti-solvent. The effects of altering five SAS operating conditions, solvent ratio, temperature, pressure, concentration of carrier, and flow rate, on the microstructure of particles were investigated using an orthogonal experimental design. Under the optimal conditions, astaxanthin/PLLA particles were produced with an encapsulation efficiency of 91.5% and a mean particle size of 954.6 nm. SEM images showed that most astaxanthin/PLLA particles were uniform microspheres. FT-IR spectra showed that the chemical structure of astaxanthin was unchanged by the SAS process. The results of chromatic difference, X-ray diffraction, thermogravimetric, and differential scanning calorimetry analyses showed that astaxanthin had been encapsulated in the PLLA matrix in an amorphous state. Overall, astaxanthin/PLLA microspheres greatly enhanced the stability of astaxanthin during storage, and the levels of residual solvents were far lower than the ICH limits. This means that astaxanthin/PLLA microspheres prepared using SAS show great potential for use in many food, cosmetic, and pharmaceutical formulations.