- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
Jinbiao Yang a 1, Tiesen Li b 1, Xiaojun Bao c, Yuanyuan Yue c *, Haiyan Liu a *
• A novel route to prepare hierarchical sodalite from a natural mineral was developed.
• Mesoporogens, post-treatment, and Si- and Al-containing chemicals were not used.
• The rapid zeolitization was performed via the reversed crystal growth route.
• The resulting sodalite had hierarchical pore structure and high basicity.
• Sodalite-derived catalysts shown excellent performance in Knoevenagel condensation.
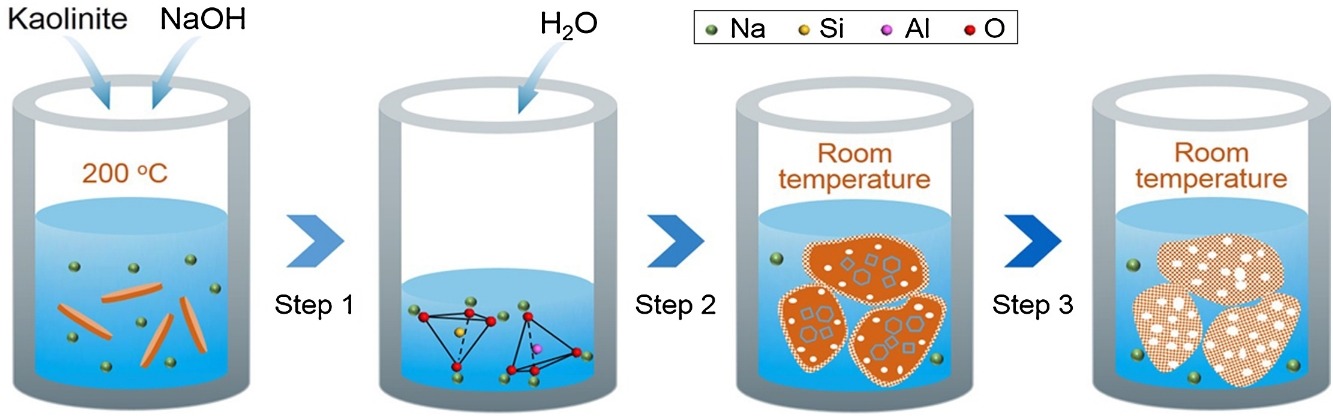
A rapid and environmentally friendly approach to synthesize hierarchical sodalite from natural aluminosilicate mineral without the involvement of any mesoporogen or post-synthesis treatment was developed. This strategy involves three important steps: the first is the depolymerization of an aluminosilicate mineral into highly reactive silicon and aluminum species with ideal meso-scale structures through activation of a sub-molten salt. The second step is the hydrolysis and condensation of the activated aluminosilicate mineral into zeolitic precursors that also have a meso-scale structure. The third is the rapid zeolitization of the zeolitic precursors through the reversed crystal growth route at room temperature and ambient pressure to form hierarchical sodalite. The physicochemical properties of the as-synthesized sodalite were systematically characterized, and the formation mechanism of the hierarchical pore structure was discussed. When used as a solid base catalyst for Knoevenagel condensation, the as-synthesized sodalite and its potassium ion-exchanged product with hierarchical micro–meso–macroporous structure both exhibited high catalytic activity and product selectivity.