- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
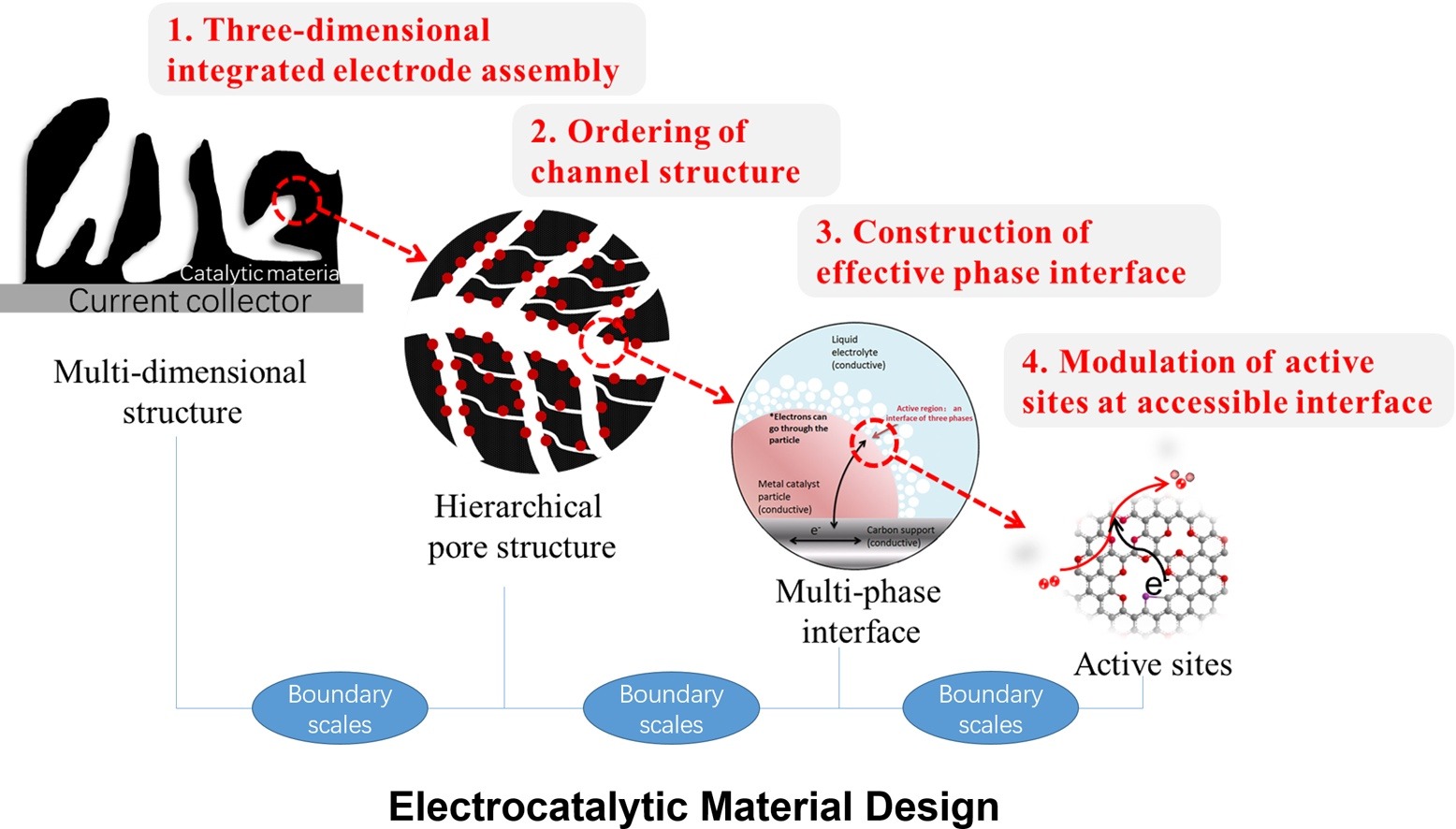
• Mesoscience is applied in electrocatalyst design for hydrogen energy conversion.
• Catalyst properties can be influenced by factors at different levels.
• Mesoscience can correlate catalytic performance with factors at different levels.
• Catalyst morphology, pore and crystal structure, size, and components are considered.
• A picture of how mesoscience can aid design of catalytic materials is provided.
Electrocatalytic materials with different morphologies, sizes, and components show different catalytic behavior in various heterogeneous catalytic reactions. It has been proved that the catalytic properties of these materials are strongly influenced by several factors at different levels, including the electrode morphology, reaction channels, three-phase interface, and surface active sites. Recent developments of mesoscience allow one to study the relationship between the apparent catalytic performance of electrocatalytic materials with these factors from different levels. In this review, following a brief introduction of new mesoscience, we summarize the effect of mesoscience on electrocatalytic material design, including modulating the geometric and electronic structures of materials focusing on morphology (particulate, fiber, film, array, monolith, and superlattice), pore structure (microporous, mesoporous, and hierarchical), size (single atoms, nanoclusters, and nanoparticles), multiple components (alloys, heterostructures, and multiple ligands), and crystal structures (crystalline, amorphous, and multiple crystal phases). By evaluating the electrocatalytic performance of catalytic materials tuned at the mesoscale, we paint a picture of how these factors at different levels affect the final system performance and then provide a new direction to better understand and design catalytic materials from the viewpoint of mesoscience.