- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
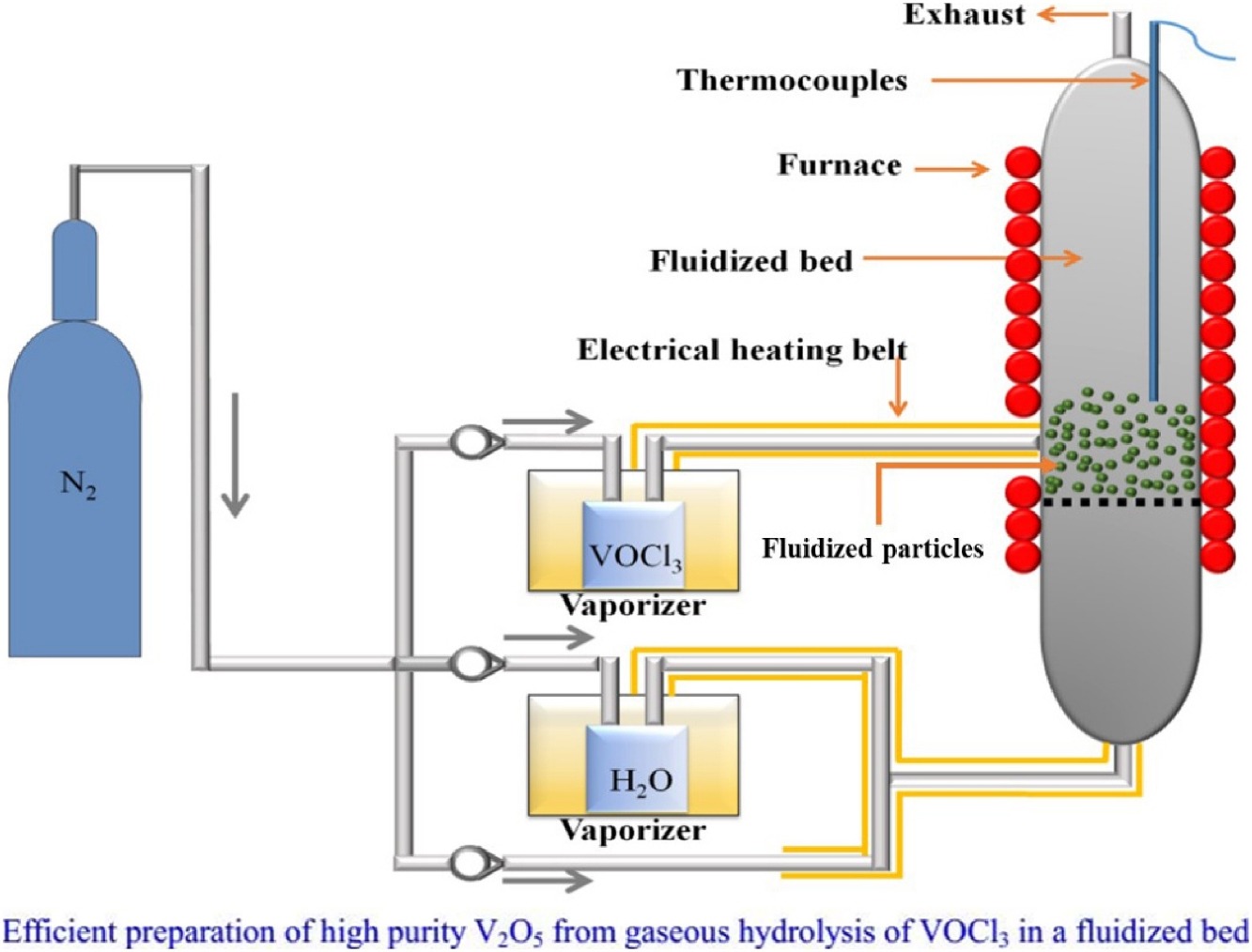
• High purity V2O5 was prepared via the gaseous hydrolysis of VOCl3 in a fluidized bed.
• Vanadium oxytrichloride (VOCl3) was used as the high purity precursor.
• A temperature of 500 °C and a H2O/VOCl3 molar ratio of 18 are optimal.
• High purity V2O5 with less than 0.05 wt% residual Cl was obtained in a yield of 85%.
Present-day all-vanadium redox flow batteries (VRFBs) generally require high purity vanadium oxide as a raw ingredient. The chlorination procedure presents distinct technical advantages with regard to preparing high purity vanadium pentoxide (V2O5) using vanadium oxytrichloride (VOCl3) as a highly pure intermediate. To efficiently prepare high purity V2O5 from VOCl3, a single-step fluidized bed chemical vapor deposition (FBCVD) method was explored in the present work. Based on thermodynamic analyses, the direct and complete conversion of VOCl3 to V2O5 is difficult, and may result in a small amount of residual Cl in the product. Consequently, the effects of temperature and the H2O/VOCl3 molar ratio on the quantity of residual Cl were assessed. The Cl concentration was found to decrease with increasing temperature or increasing H2O/VOCl3 molar ratios. Additionally, Cl was determined to be present only in the form of Clsingle bondV bonds, while Clsingle bondH and Clsingle bondCl bonds were not detected in a V2O5 product made at 200 °C with a H2O/VOCl3 molar ratio of 18. A Cl concentration of less than 0.05 wt% was obtained under the optimal synthesis conditions, demonstrating that the FBCVD method is a viable means of preparing high purity V2O5 via the gaseous hydrolysis of VOCl3.