- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
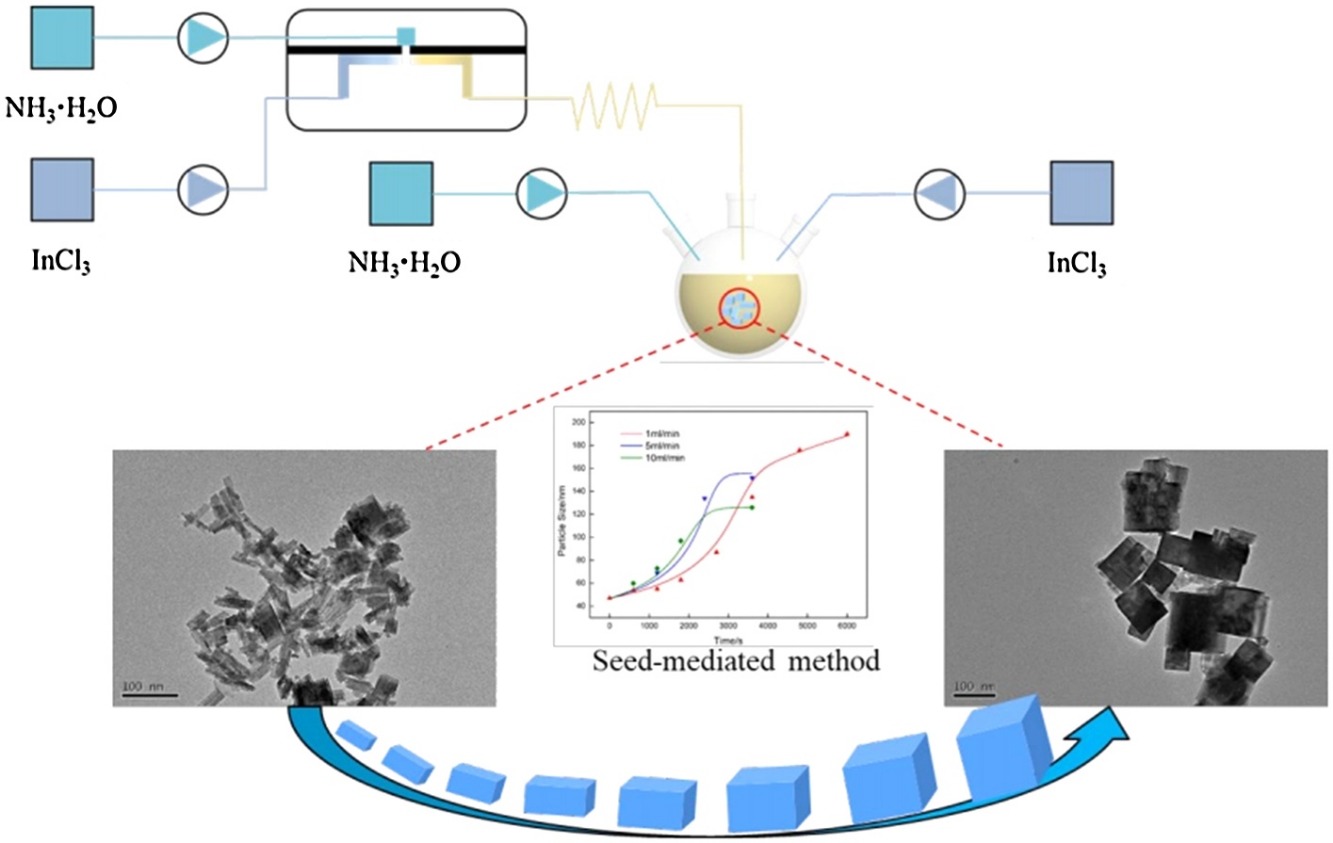
- In(OH)3 and In2O3 particles through a seed-mediated growth method in microreactor were synthesized.
- Seed-mediated particles were larger than those synthesized by precipitation method.
- Particles size increased with concentrations and decreased with flow rates.
- A growth model consistent with the experimental results was established.
Indium hydroxide (In(OH)3) and indium oxide (In2O3) particles are typically synthesized through chemical precipitation methods. In this study, we used a seed-mediated growth method and microreactor-based synthesis process. We synthesized cubic In(OH)3 particles with a crystal size of 172 nm from an 5% (w/v) indium chloride solution. The In2O3 particles synthesized through the thermal decomposition of In(OH)3 particles featured crystals up to 90 nm in size with an average size of 73 nm, which were much larger than the 20–30 nm In2O3 particles synthesized by a traditional precipitation method. The concentrations of the seed and growth solutions were varied from 1% to 7% (w/v). The crystal size of the particles increased with the concentration of the seed and growth solutions; this tendency was the opposite to that observed for the precipitation method. Through the use of a 5% (w/v) seed solution, the flow rate of the growth solution was varied from 1 to 10 mL/min, and the resulting crystal size decreased as the flow rate was increased. To understand the reasons for this trend, the growth rate of the crystals was determined at different flow rates (i.e., 1, 5, and 10 mL/min). A growth model consistent with the experimental results was established, which demonstrated that slow addition of the growth solution was beneficial for preparing large indium hydroxide particles.