- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
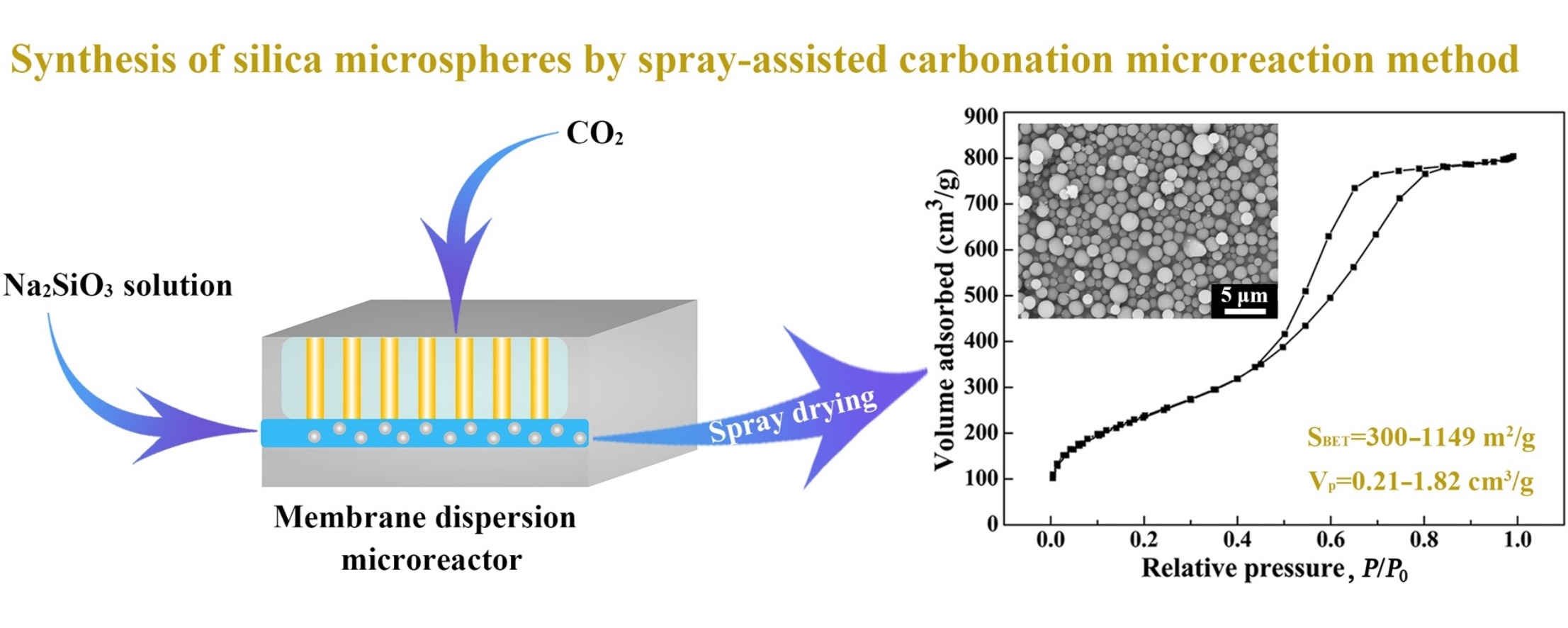
• Spray-assisted carbonation method to prepare silica microspheres is first reported.
• Synthetic process includes the preparation of a silica sol, gelation, and spray drying.
• Carbonation reaction was conducted in a microreactor with Na2SiO3 as the silica source.
• Mesoporous silica microspheres exhibit excellent dispersity, with diameters of 1–2 μm.
• This method can be extended to prepare various silica materials and related composites.
A novel spray-assisted carbonation microreaction method for the synthesis of mesoporous silica microspheres is reported. The synthetic process comprises the preparation of a silica sol via a carbonation reaction, rapid gelation at high temperature, and subsequent rapid solvent evaporation by spray drying. The carbonation microreaction was conducted in a membrane dispersion microreactor, in the presence of sodium silicate and carbon dioxide reactants. The as-synthesized silica microspheres exhibit a uniform mesostructure, excellent dispersity, and a narrow particle size distribution, with average diameters of 1–2 μm, Brunauer–Emmett–Teller surface areas of 300–1149 m2/g, and total pore volumes of 0.21–1.82 cm3/g. Relatively low concentrations of the silicate species and well-controlled silica condensation rates are responsible for the formation of the observed spherical morphology. The synthetic process is of significant practical importance as a result of using low-cost raw materials, and because of the excellent controllability and process stability displayed. Furthermore, this rapid and flexible method may be extended to the synthesis of various silica materials and their composites.