- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
• Fluidization behavior and reducibility of various iron ore grades were investigated.
• A stable fluidization of hematite- and limonite-based iron ores was observed.
• The magnetite-based iron ore could not be fluidized during reduction.
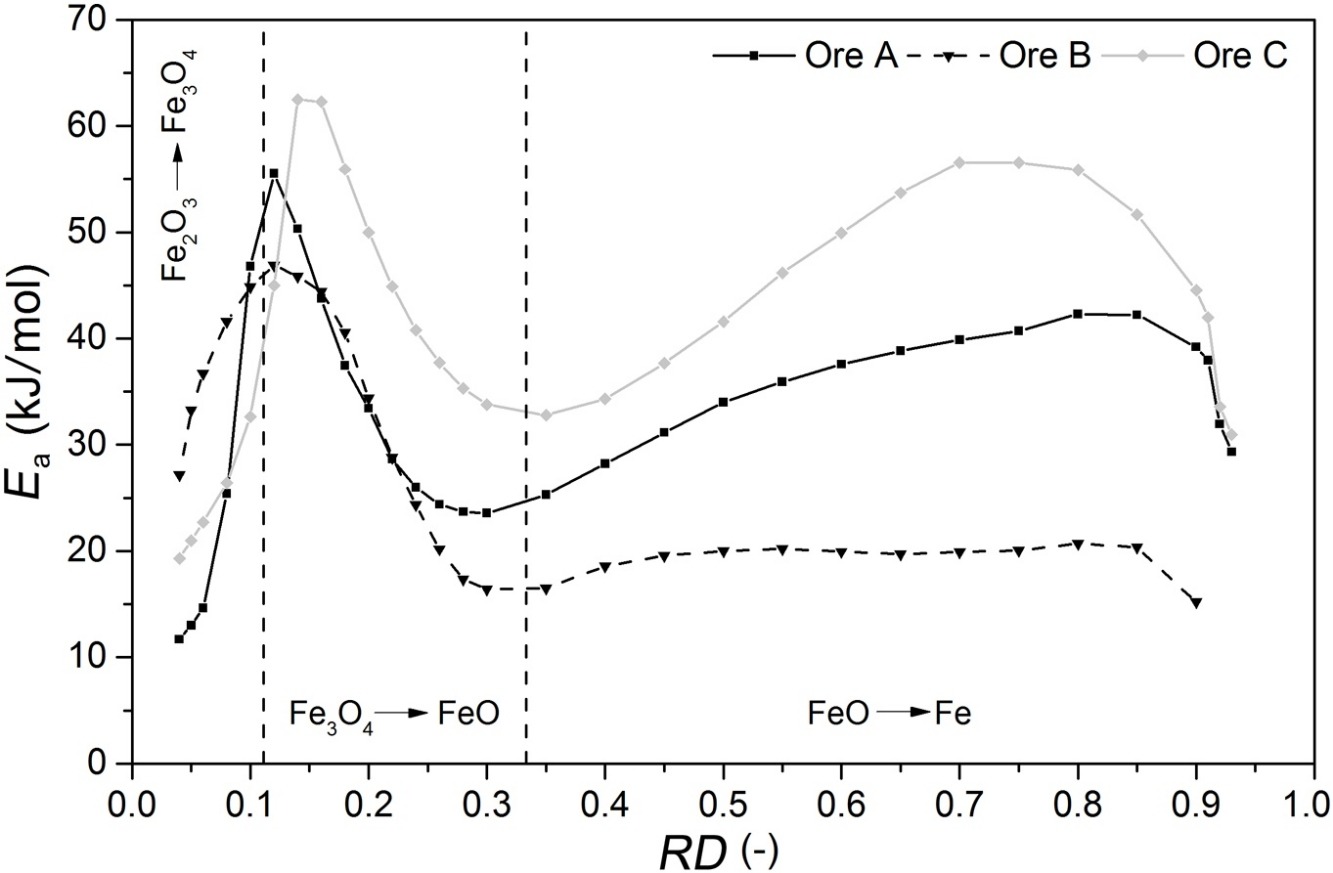
• Trends of apparent activation energies varied as a function of reduction.
A laboratory fluidized bed reactor was used to investigate the fluidization behavior and reducibility of various iron ore fines. Hydrogen was chosen as a reducing agent across a temperature range of 873–1073 K. The magnetite ore used exhibited strong sticking behavior after the initiation of metallic iron formation. All other tested ores fluidized sufficiently well when subjected to the same high reduction temperatures. Parallel kinetic analysis was conducted using a previously developed model to include three rate-limiting step types. The trend of apparent activation energy was correlated with the degree of reduction. Additionally, the influence of varying the specific gas rate was investigated. The results show the variation in reducibility as a result of different interactions, which influence the rate-limiting mechanisms of nucleation and the undertaken chemical reactions, which vary as a function of temperature and degree of conversion. The apparent activation energies, determined from the reduction of wüstite to metallic iron, were in the range of 15–60 kJ/mol, depending on the iron ore used and the degree of conversion. The change in apparent activation energy deriving from the increased specific gas rate can be explained by an increasing nucleation effect, especially at lower reduction temperatures.