- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
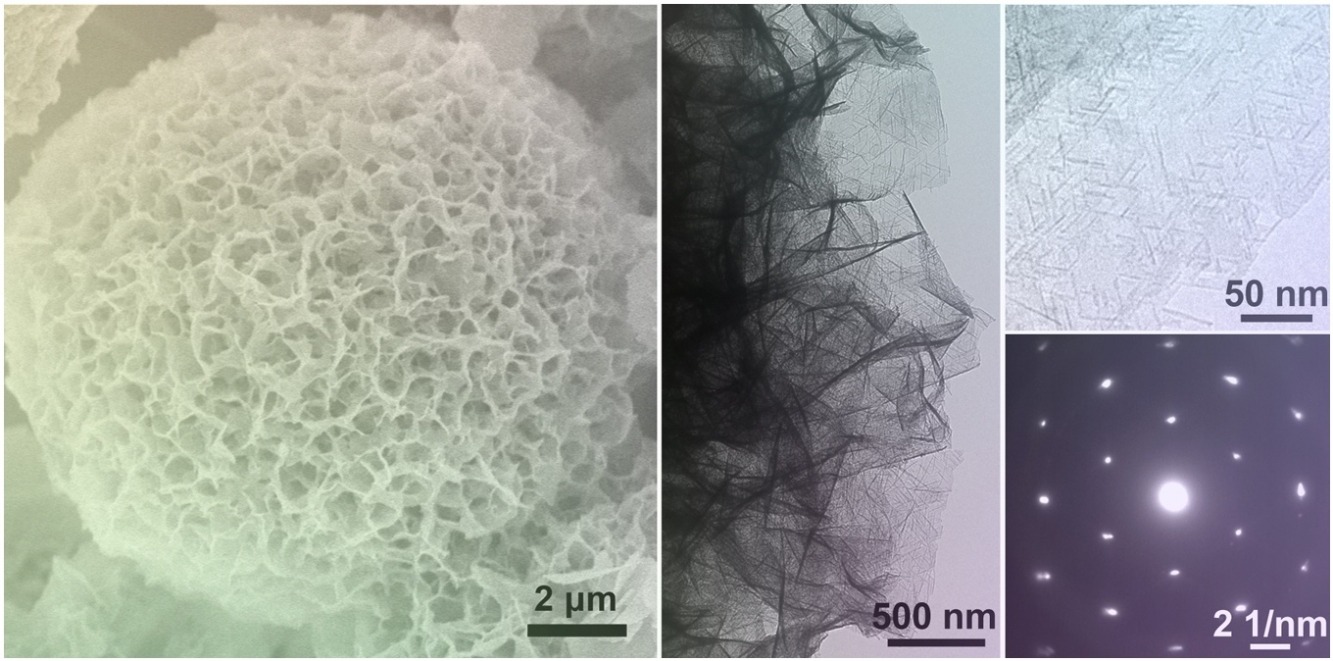
• 1D-2D-3D hierarchical nanostructure with rich edge sites for facile pre-oxidation.
• Medium-concentration La dopant inducing enriched local Ni3+ ions.
• Good OER and UOR activity with low overpotential and large current density.
• Enhanced pre-oxidation improving operational stability and activity.
The development of advanced electrocatalysts for electro-oxidation reactions has attracted much attention because of the critical role of such electrocatalysts in improving the overall efficiency of coupled hydrogen production. We have developed an efficient lanthanum-doped α-Ni(OH)2 bifunctional catalyst with a 1D-2D-3D hierarchical nanostructure. It shows superior activity and stability in the anodic oxygen evolution reaction (OER) and urea oxidation reaction (UOR). Enrichment of the edge sites and conducting La doping in α-Ni(OH)2 phase enable formation and stabilization of abundant local Ni3+ ions. This guarantees ultralow onset potentials in electro-oxidation reactions. The 1D-2D-3D hierarchical nanostructure significantly boosts the in situ generation of high-valence active species, which results in efficient and stable OER and UOR performances, and the synergistic merit of doping-induced facile reaction kinetics. Because of the structural benefits of a large surface area, charge-transfer promotion, good structural stability, and bifunctionality, a 1% La-doped α-Ni(OH)2 hierarchical nanostructure gives superior OER and UOR performances with low overpotentials, large catalytic current densities, and excellent operational stability. It is therefore a promising catalyst for use in simultaneous alkaline wastewater treatment and hydrogen production.