- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
• Different solid phases with new thermodynamic, mechanical, and kinetic properties.
• Promising results on in vitro/in vivo were highlighted.
• Nicotinamide and derivatives are by far the most preferred choice of co-formers.
• LAG has shown to be upper to solvent-free methods on NSAIDs co-crystals.
• Some phase III clinical trials show greater analgesic and anti-inflammatory activity.
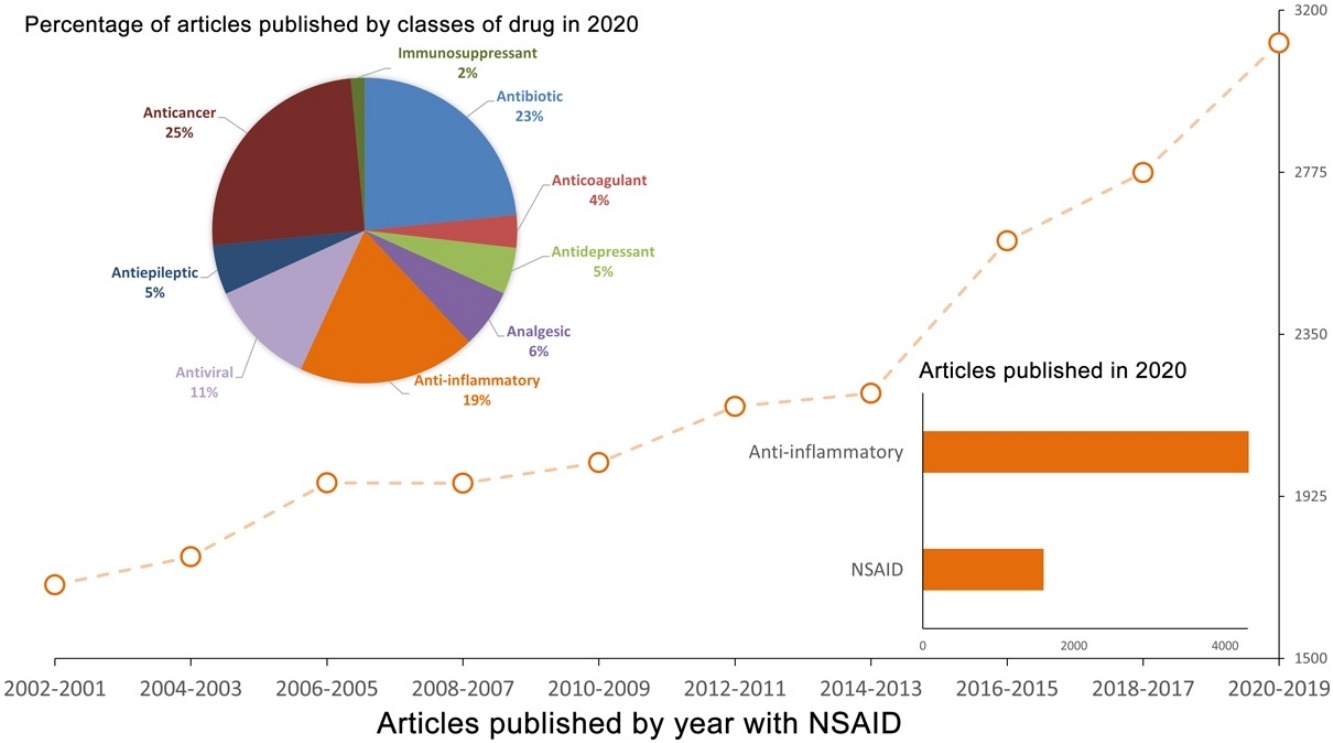
Pharmaceutical co-crystals have been explored by many researchers as a strategy to optimize physicochemical properties of solid-state drugs. Their improvements of solubility, bioavailability, and the reduced tendency for phase transformation occurrence, are factors that highlight benefits of pharmaceutical co-crystals among other solid forms. According to the Biopharmaceutical Classification System (BCS), non-steroidal anti-inflammatory drugs (NSAIDs) are class II drugs, which have low aqueous solubility and therefore co-crystallization has the potential to optimize NSAID product properties. In this review, we highlight the recent progress made on NSAIDs co-crystals, their co-formers, synthesis, methods and use, while we underline some promising results on in vitro and in vivo co-crystal properties. A celecoxib-tramadol co-crystal reaches phase III clinical trials, showing greater analgesic activity than both individual APIs. The aqueous solubility of the co-crystal formed between l-proline and diclofenac is very high in comparison with the pure drug. Naproxen co-crystals with urea and thiourea have an increase of drug release of almost 60%. Co-crystal design brings a new perspective in drug development since the co-former used can also be a biologically active component allowing to combine different anti-inflammatory drugs, which have an incredible spectrum of application due to the analgesic, anti-pyretic and anti-inflammatory properties.