- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
Guangxing Yang a b, Qiao Zhang c, Hao Yu a b, Feng Peng c *
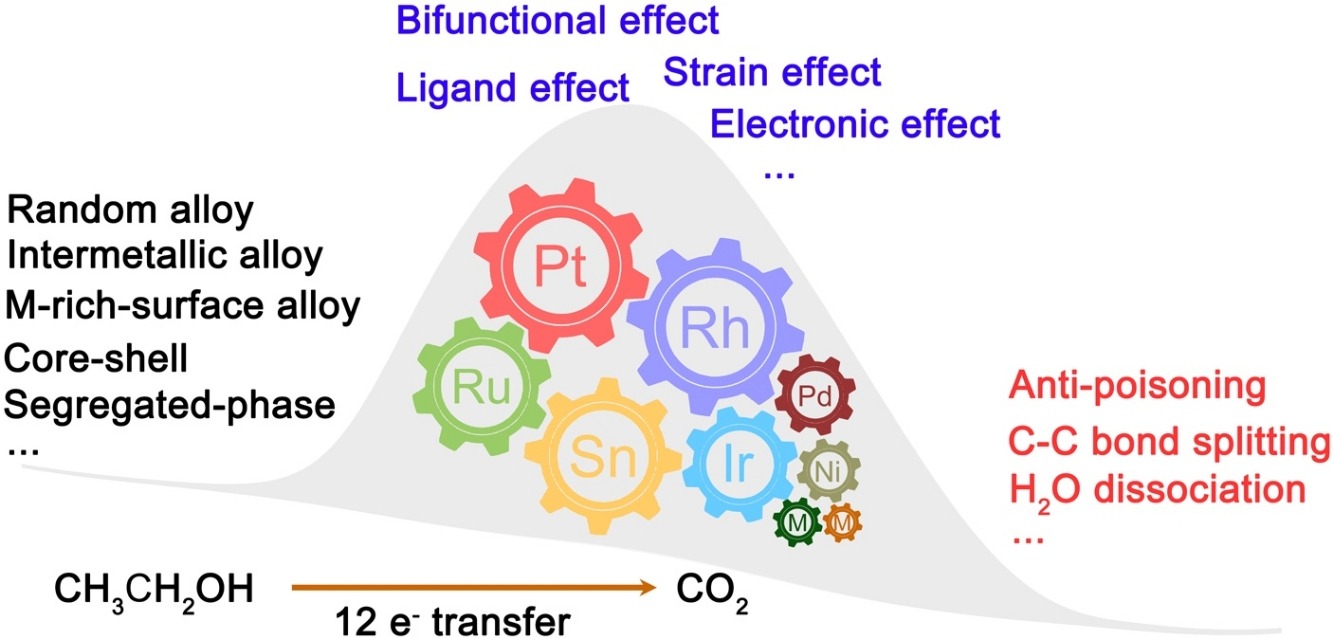
• Direct ethanol fuel cells are promising devices, attracting increasing attention.
• Pathways of ethanol oxidation reaction (EOR) to C1 and C2 species were analyzed.
• Most ternary catalysts for EOR devised over the last fifteen years are summarized.
• Structure-composition-reactivity relationships of ternary catalysts in EOR are discussed.
Direct ethanol fuel cell (DEFC) as a promising device for converting chemical energy to electricity has been paid ever-increasing attention. However, the slow kinetics of ethanol electrooxidation at an anode hinders the application of DEFCs. Although Pt is the best catalyst among all the pure metal catalysts, it still has a relatively poor ability to break the Csingle bondC bond, is deactivated by the accumulated COad intermediates, and undergoes unwanted desired structure change over long-term operation. In recent years, the addition of other metals to form binary, ternary, and quaternary catalysts have significantly improved electroactivity and stability. Ternary catalysts can have numerous element combinations and complicated architectures and, therefore, have been the subject of considerable research. In this review, most of the reported ternary catalysts will be summarized and categorized according to their structure while discussing the essence of the role of each component.