- Volumes 84-95 (2024)
-
Volumes 72-83 (2023)
-
Volume 83
Pages 1-258 (December 2023)
-
Volume 82
Pages 1-204 (November 2023)
-
Volume 81
Pages 1-188 (October 2023)
-
Volume 80
Pages 1-202 (September 2023)
-
Volume 79
Pages 1-172 (August 2023)
-
Volume 78
Pages 1-146 (July 2023)
-
Volume 77
Pages 1-152 (June 2023)
-
Volume 76
Pages 1-176 (May 2023)
-
Volume 75
Pages 1-228 (April 2023)
-
Volume 74
Pages 1-200 (March 2023)
-
Volume 73
Pages 1-138 (February 2023)
-
Volume 72
Pages 1-144 (January 2023)
-
Volume 83
-
Volumes 60-71 (2022)
-
Volume 71
Pages 1-108 (December 2022)
-
Volume 70
Pages 1-106 (November 2022)
-
Volume 69
Pages 1-122 (October 2022)
-
Volume 68
Pages 1-124 (September 2022)
-
Volume 67
Pages 1-102 (August 2022)
-
Volume 66
Pages 1-112 (July 2022)
-
Volume 65
Pages 1-138 (June 2022)
-
Volume 64
Pages 1-186 (May 2022)
-
Volume 63
Pages 1-124 (April 2022)
-
Volume 62
Pages 1-104 (March 2022)
-
Volume 61
Pages 1-120 (February 2022)
-
Volume 60
Pages 1-124 (January 2022)
-
Volume 71
- Volumes 54-59 (2021)
- Volumes 48-53 (2020)
- Volumes 42-47 (2019)
- Volumes 36-41 (2018)
- Volumes 30-35 (2017)
- Volumes 24-29 (2016)
- Volumes 18-23 (2015)
- Volumes 12-17 (2014)
- Volume 11 (2013)
- Volume 10 (2012)
- Volume 9 (2011)
- Volume 8 (2010)
- Volume 7 (2009)
- Volume 6 (2008)
- Volume 5 (2007)
- Volume 4 (2006)
- Volume 3 (2005)
- Volume 2 (2004)
- Volume 1 (2003)
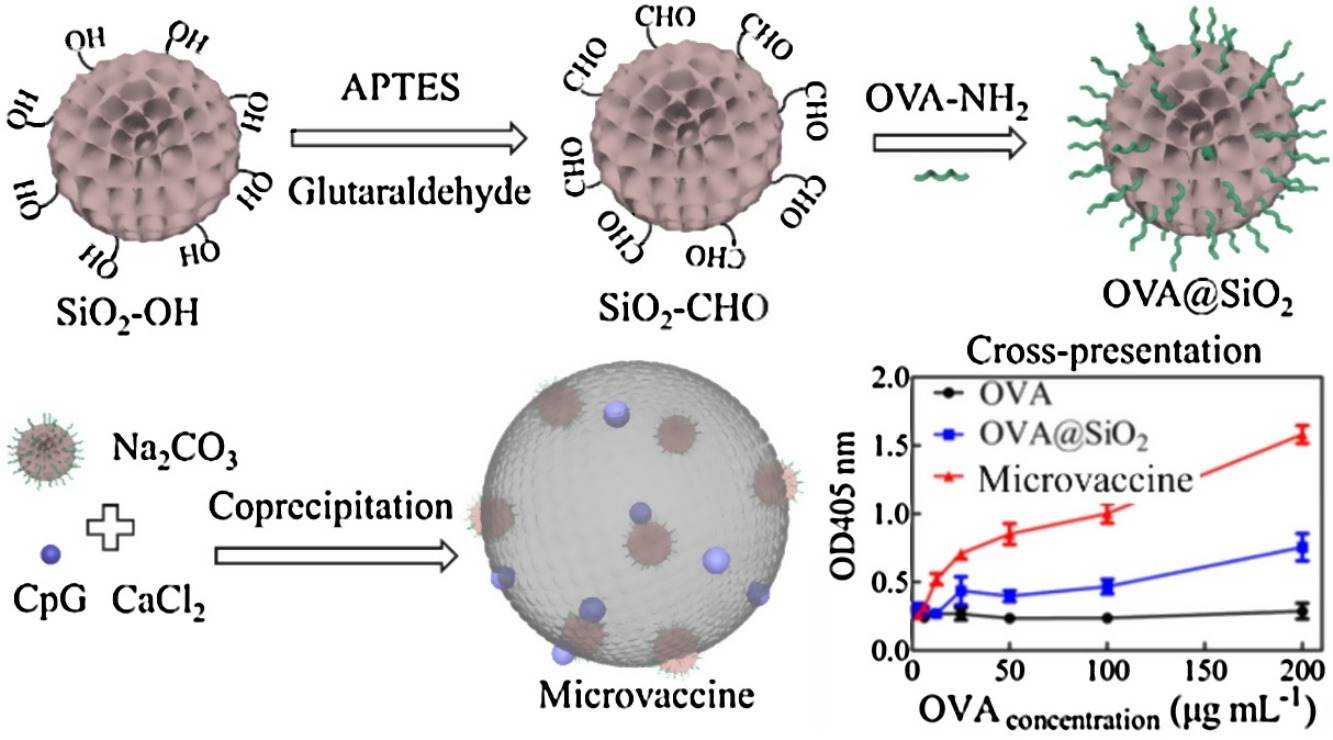
• A microvaccine with SiO2 nanoparticles in CaCO3 microparticles was constructed.
• Antigen was conjugated with SiO2, then SiO2 and CpG were co-encapsulated into CaCO3.
• Vaccine was internalized via micropinocytosis and improved antigen/adjuvant uptake.
• Antigen lysosomal escape was observed, and DCs pulsed by vaccine were fully mature.
• The vaccine had good biocompatibility and protected antigens from rapid degradation.
A microscale vaccine containing SiO2 nanoparticles loaded in CaCO3 microparticles was constructed using the co-precipitation method. The antigen ovalbumin (OVA) was covalently conjugated with SiO2 nanoparticles, and these nanoparticles and CpG were co-encapsulated into CaCO3 microparticles, generating a vaccine with a size of approximately 5.2 μm. Scanning electron microscopy (SEM), energy-dispersive X-ray (EDX), elemental mapping, and Fourier transform infrared (FTIR) analyses confirmed the successful preparation of the microscale vaccine; the vaccine had good storage stability without sustained antigen release, and negligible cytotoxicity to dendritic cells (DCs) and macrophages. Compared to SiO2 nanoparticles, the microscale vaccine can significantly improve antigen/adjuvant uptake. DCs internalized the entire microscale vaccine into lysosomes via macropinocytosis, and an increase in antigen endo/lysosomal escape was observed by confocal laser scanning microscopy (CLSM). Specifically, DCs pulsed with the vaccine were fully mature, expressing high levels of costimulatory molecules (CD40, CD80, and CD86), MHC II, and MHC I and secreting high levels of proinflammatory cytokines (IL-12, TNF-α, IL-1β, and IL-6). In addition, the vaccine had good in vivo biocompatibility, could protect the antigen from rapid degradation, and increased the retention time in lymph nodes. SiO2 nanoparticles-in-CaCO3 microparticles were an excellent carrier for antigen and adjuvant delivery. Hopefully, this study can provide some information on the design of microscale carriers for vaccine delivery systems.